The graph of compressibility factor (Z) vs. P for one mole of a real gas ..
By A Mystery Man Writer
Solution For The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very hi
The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very high pressure (dPdZ) is (2.81)atm−1 , then calculate volume of one mole of real gas molecules (in L/mol) Given : NA=6×1023 and R=27322.4LatmK−1mol−1
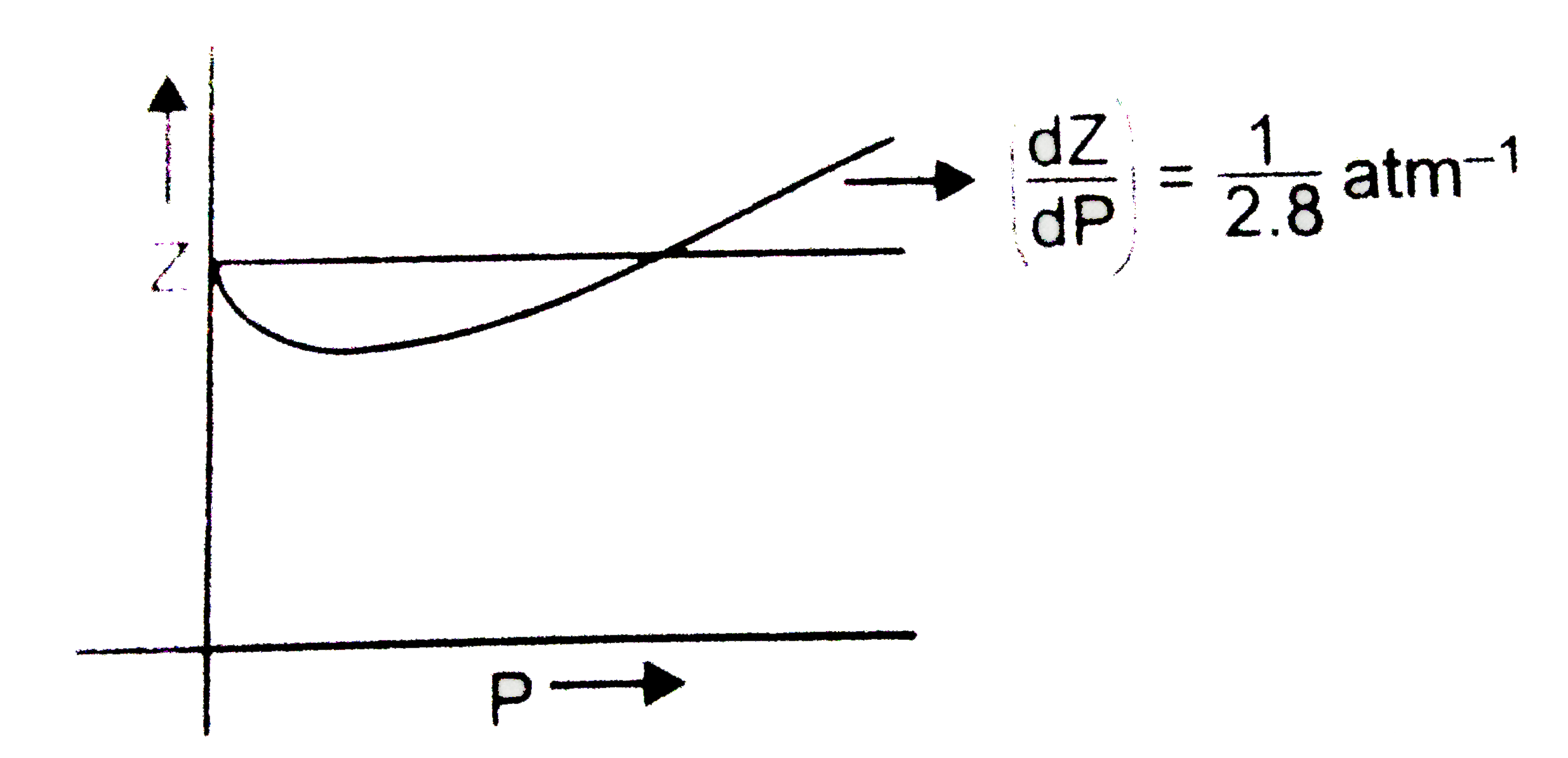
The graph of compressibility factor (Z) vs. P for one mole of a real g

For one mole of a real gas, curves are plotted under different

11-06-23 - JR - Iit - Star Co-Sc (Model-A) - Jee Adv - 2014 (P-I
The given graph represents the variations of compressibility

Compressibility Factor Calculator - File Exchange - MATLAB Central

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for
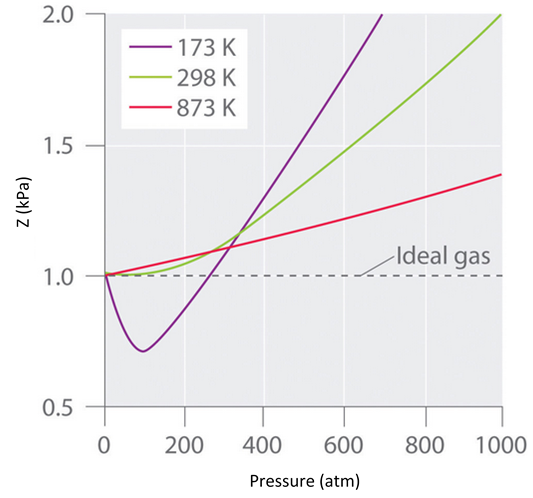
4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry
Punjabi] The graph of compressibility factor (Z) vs. P for one mole o

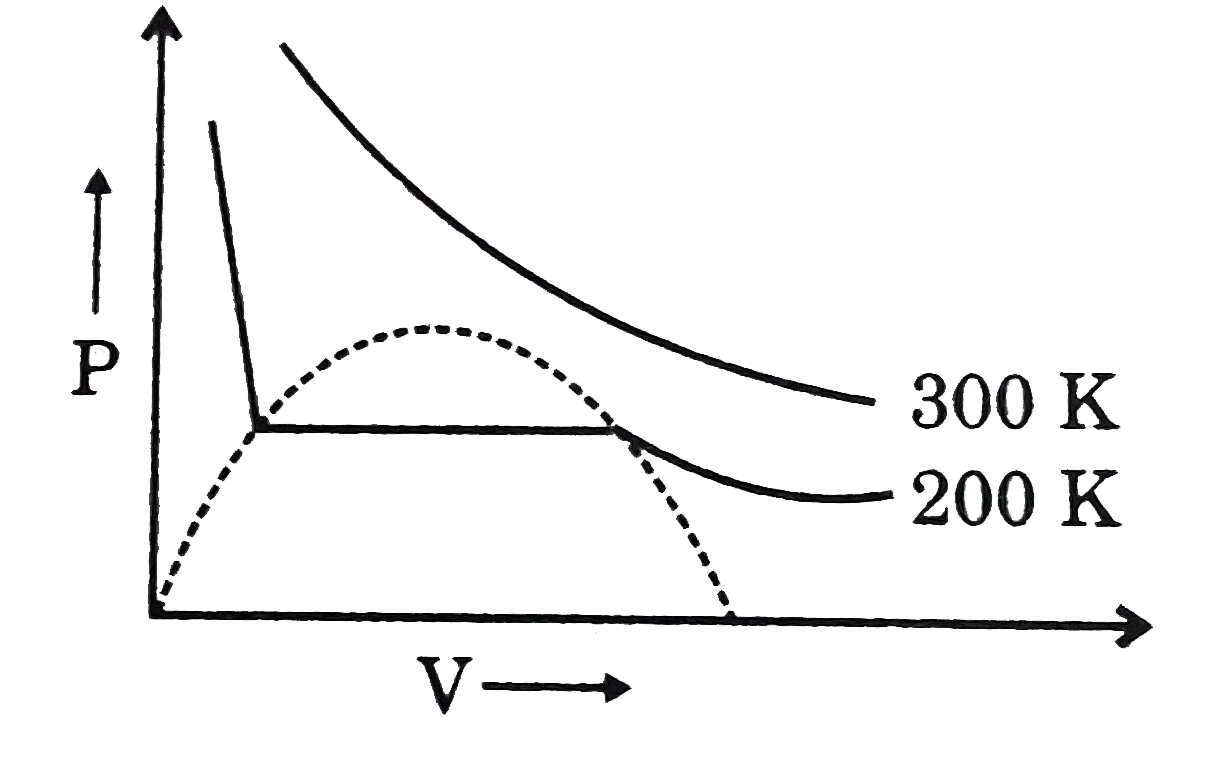
Gaseous State, PDF, Gases

Gas compressibility factor Z: Ideal gas vs Real gas
- Girl's Athletic Leggings with Pockets Youth Compression Dance Tights Yoga Pants No Front Seam Black 12

- K603 E Mens Brazilian tanga Thong Narrow waist half-back Rio back

- Corset Jean Paul Gaultier Black size 44 IT in Synthetic - 32594523

- How Often Should You Wash Your Clothes? - Organize and Decorate

- Swimsuits For All Women's Plus Size Confidante Bra Sized Underwire Bikini Top, 44 Dd - Pink Boho Paisley : Target





