What is the compressibility factor (Z) for 0.02 mole of a van der

By A Mystery Man Writer

Compressibility factor (gases) - Citizendium

Efficient estimation of natural gas compressibility factor using

The compressibility factor for nitrogen at 330 K and 800 atm is 1.90 and at 570 K and 200 atm

Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift

Compressibility factor (Z) for a van der Waals real gas at critical point is
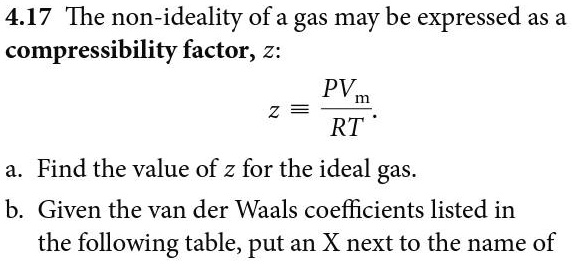
SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der

Reascon (R) Even at low pressures, repulsive forces dominate in hydrogen ..

0.585%NaCl solution at 27∘C has osmotic pressure of

Poulduly 59. What is the compressibility fac is the compressibility factor (Z) 0.02 mole co Vanderwaals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. . RT =

Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Simulations of Micellization of Sodium Hexyl Sulfate
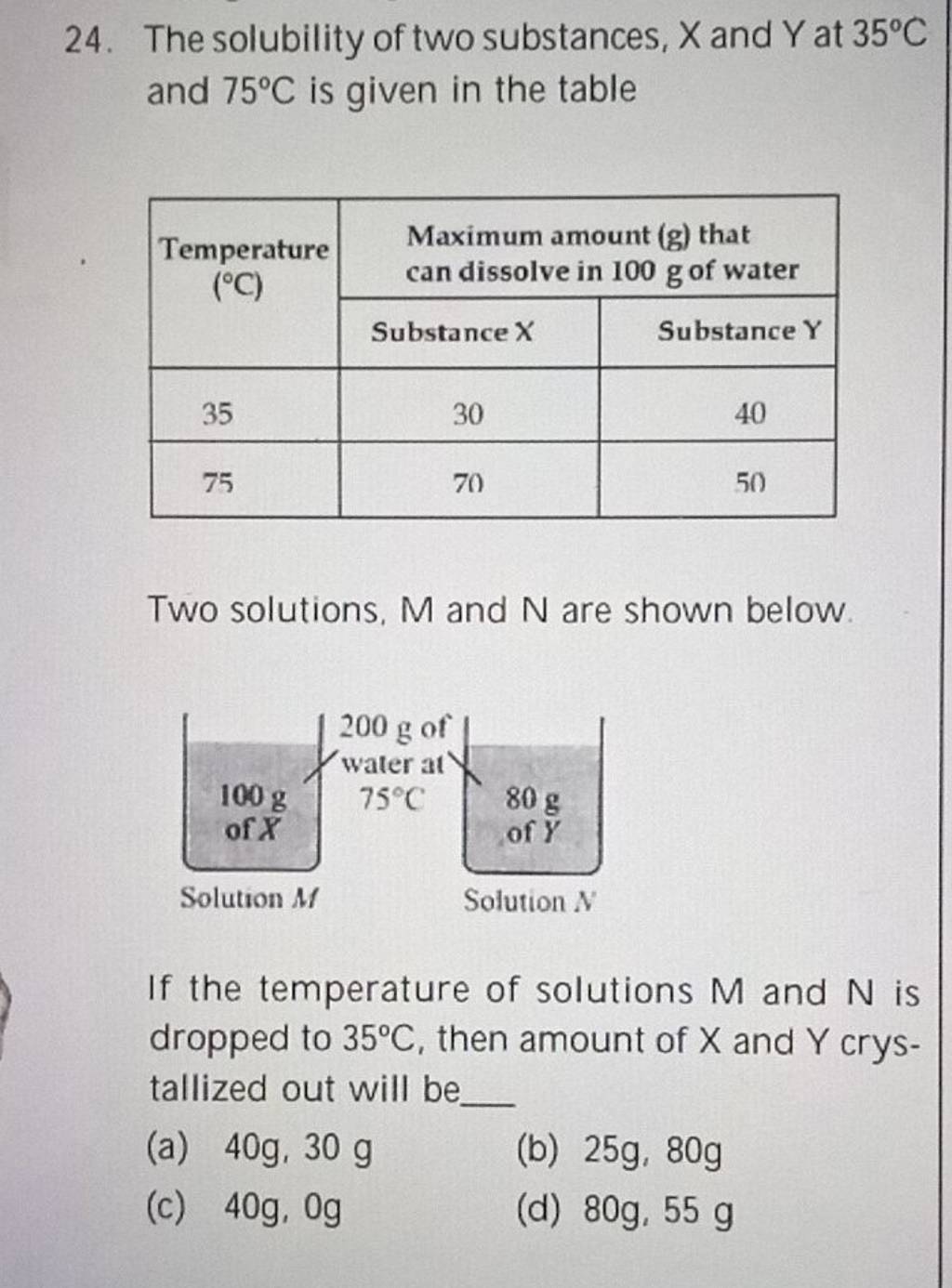
The solubility of two substances, X and Y at 35∘C and 75∘C is given in th..
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- 3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

- Math cad compressibility factor, z, of real gas using the redlich
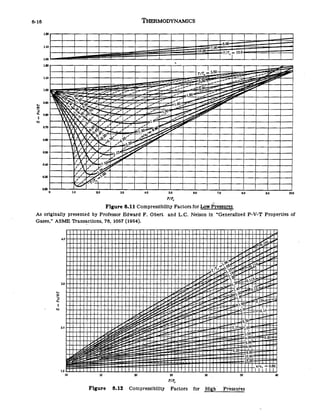
- Compressibility Factor, Z, for Various Methods.

- Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver



/product/39/060639/1.jpg?6390)

