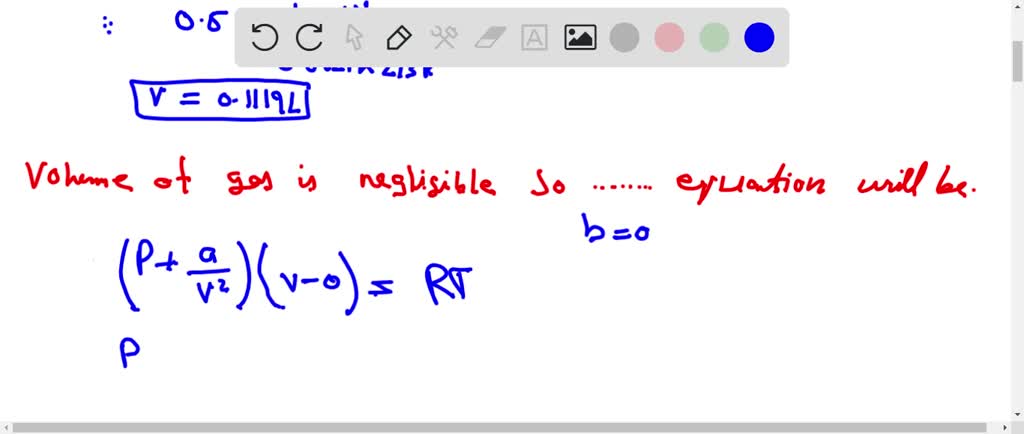
The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a
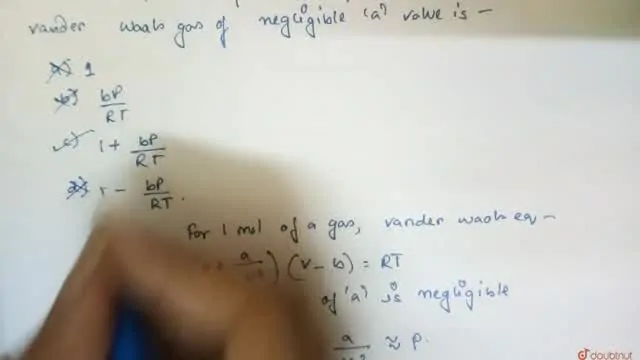
Bengali] The compresibility factor (Z) of one mole of a van der waals
⏩SOLVED:The compression factor (compressibility factor) for one mole…

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

A. The compression factor tox 1 mol of vandere waal gas _0°C and 100 ath pressure is found to 0.5. Assume that Value of gas molecule is heyligible, calculate the bandes waal
6.3: Van der Waals and Other Gases - Physics LibreTexts

The compression factor (compressibility factor) for one mole of a v

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at
Between N2 and O2, which gas is more ideal? - Quora
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
- Solved Real gas effects can be expressed as departures from

- Compressibility Factor - an overview

- Which of the following statements is/are correct? (a) all real gases are less compressible

- PPT - GASES PowerPoint Presentation, free download - ID:2088317

- If Z is a compressibility factor, van der Waals equation at low

- Customized Polos, Logo Polo Shirts, Casual Uniform Shirts, Casual Uniform Shirts, Casual Uniform Polo Shirts, Casual Uniform Polo Shirts
- MAGIC Las Vegas: Sustainability, tech on display for 2022 show

- Cool Gothic Rings Set for Women Men Girls, Vintage Silver Goth Punk Rings Bulk

- Burgundy Velvet Blazer

- SHEKINI Women Bras and Panty Sets Lace Lingerie Set Underwire Matching Bra and Thong Sets for Women 2 Piece

