If Z is a compressibility factor, van der Waals equation at low

By A Mystery Man Writer
Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Compressibility factor (z): real gases deviate from ideal behav-Turito
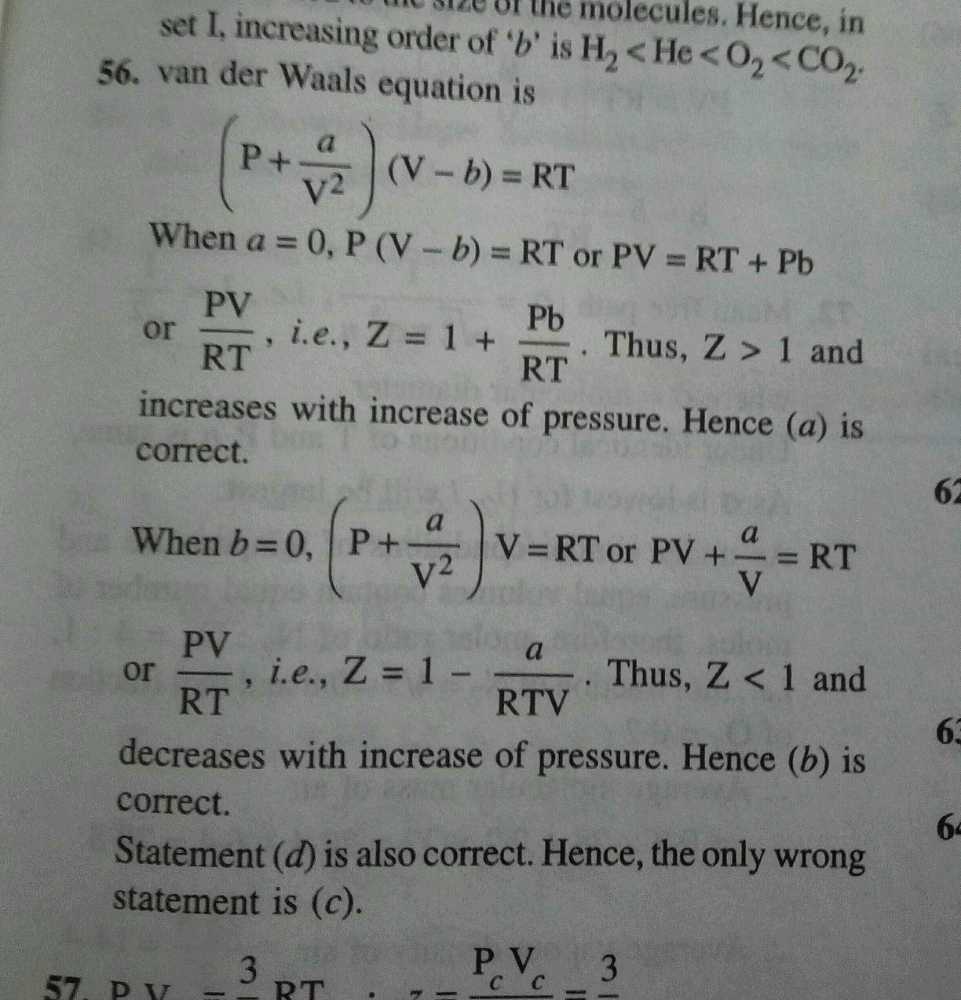
The given graph represents the variation of Z (compressibility factor =) versus P, for three real gases A, B and C. Identify the only incorrect statement. [JEE 2006]a)For the gas A, a = 0 and its dependence on P is linear at all pressureb)For the gas B, b =


If Z is a compressibility factor, van der Waals' equation at low

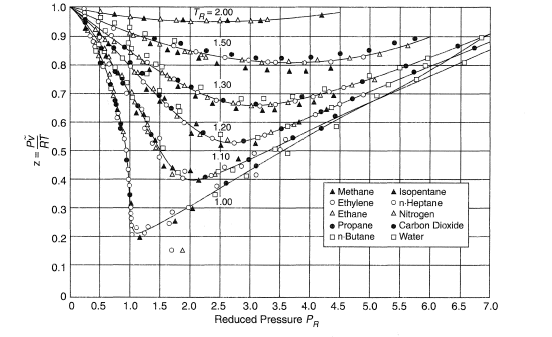
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

If `Z` is a compressibility factor, van der Waals' equation at low
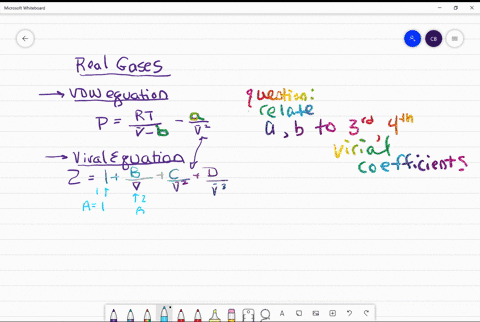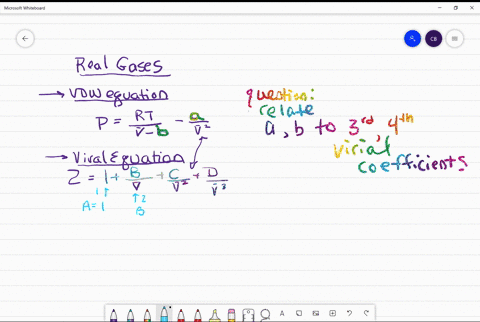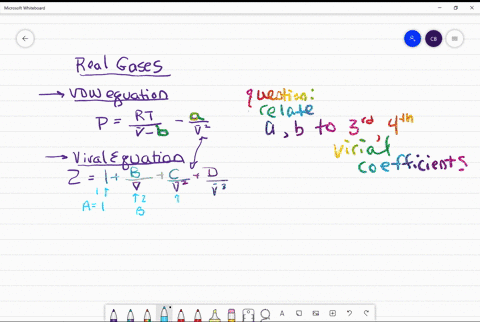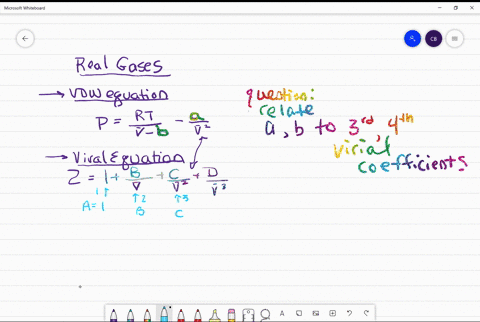
⏩SOLVED:Use the van der Waals constants for CH4 in Table 1.3 to
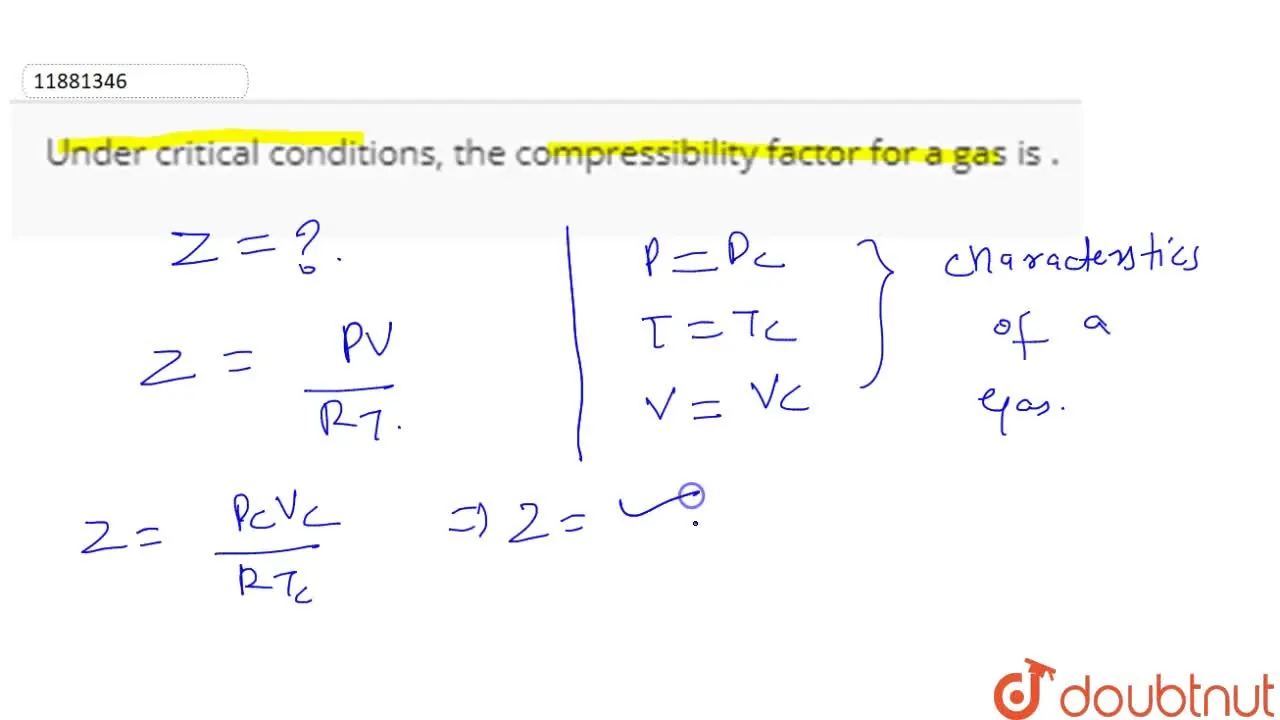
Under critical conditions, the compressibility factor for a gas is .
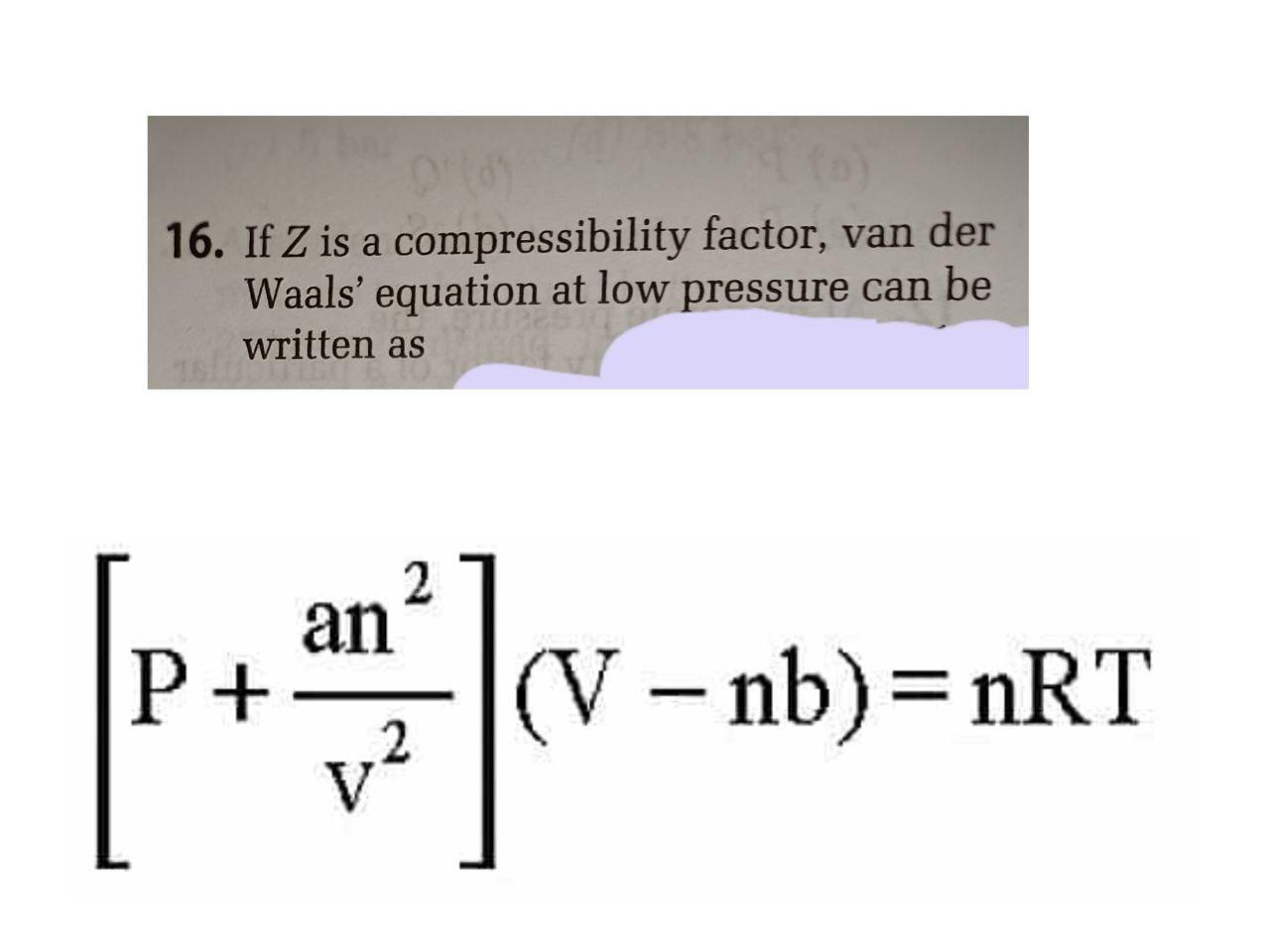
Solved 16. If Z is a compressibility factor, van der Waals
GAS LAW
- Gas Compressibility Factor Calculator Excel SpreadsheetLow Cost Easy to Use Spreadsheets for Engineering Calculations Available at Engineering Excel Spreadsheets
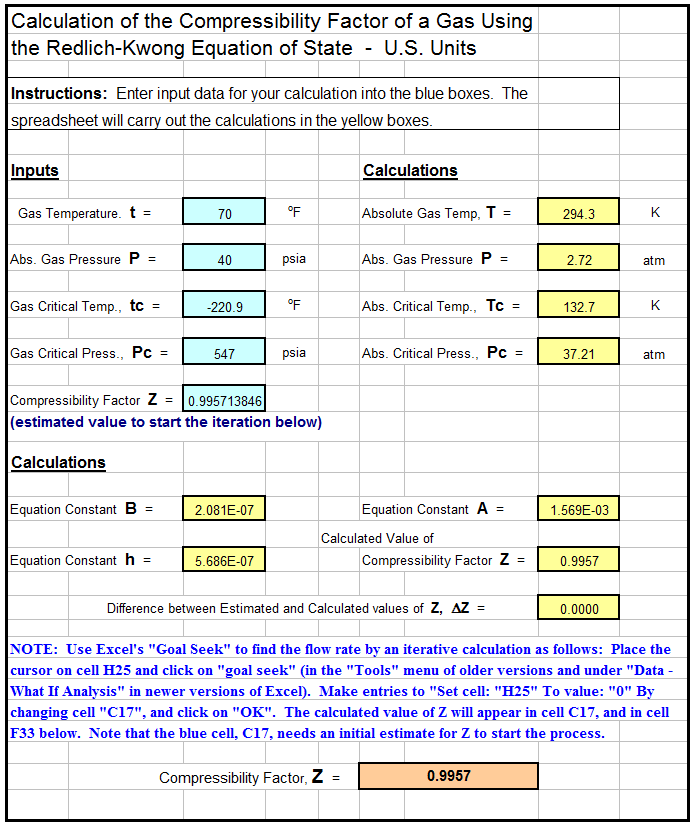
- Compressibility Factor Z
- Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1
- Math cad compressibility factor, z, of real gas using the redlich-kwong equation of state

- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
- Nike Pro Girls' Sports Bra. Nike AU

- Space: A Nonfiction Companion to Magic Tree House #8: Midnight on the Moon (Magic Tree House (R) Fact Tracker #6) (Paperback)

- When was the Queen's Silver Jubilee and how old was she? – The Sun

- Zivame - ✨✨Show Off Those Straps & Let Your Glam Shine
- Gorteks Sara long sleeve bodysuit pink powder pink


