Compressibility factor (Z) for a van der Waals real gas at

By A Mystery Man Writer
Share your videos with friends, family and the world

Real Gases and the van der Waals Equation Explained

COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

If Z is a compressibility factor, van der Waals equation at low pressure can be written as [JEE

Behaviour of Real Gases, PDF, Gases
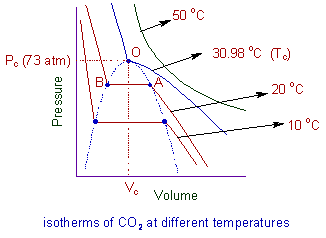
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
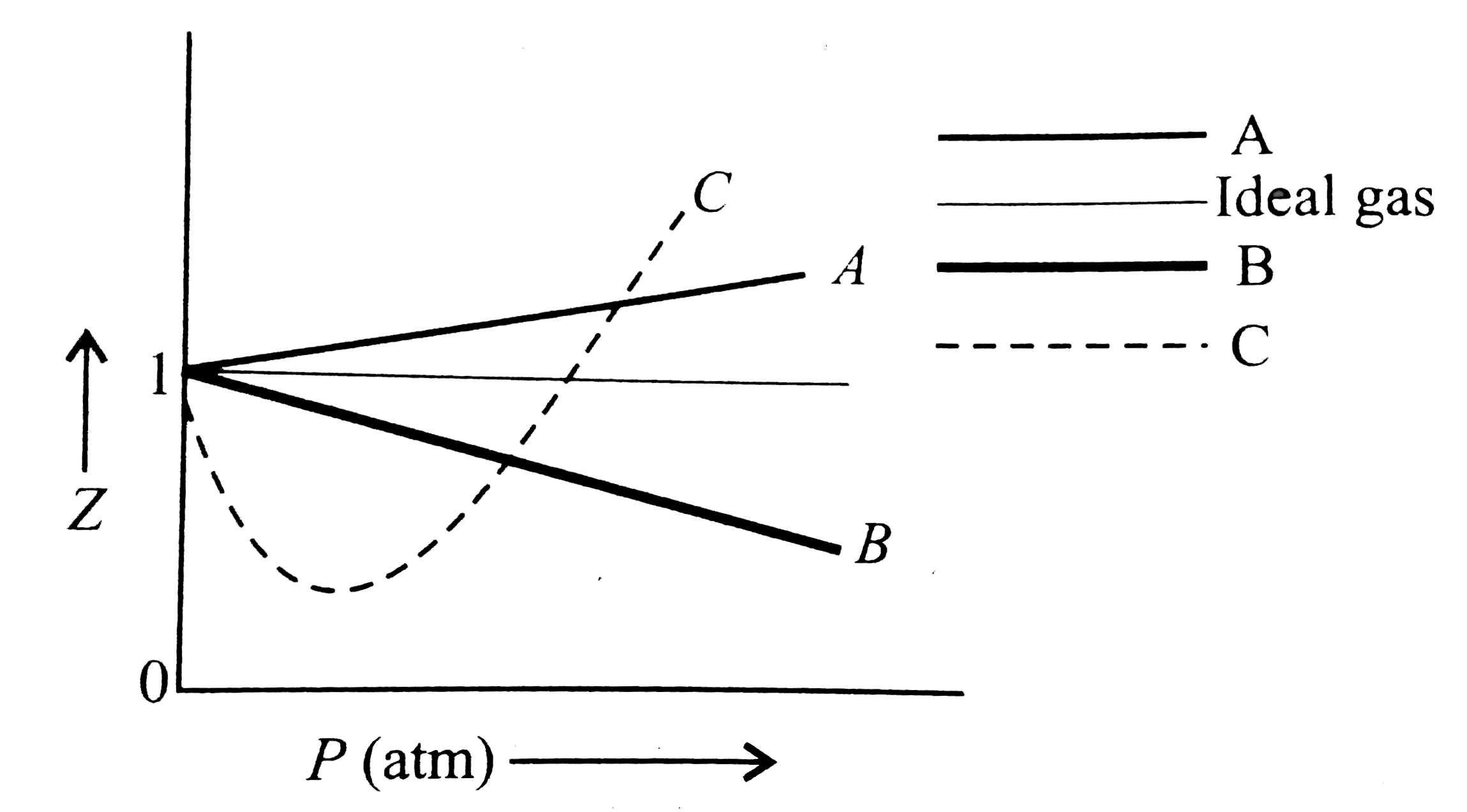
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

The, compressibility factor (Z) of one mole of a van der waals gas of negligible a value is: a.

Compressibility Factor Calculator
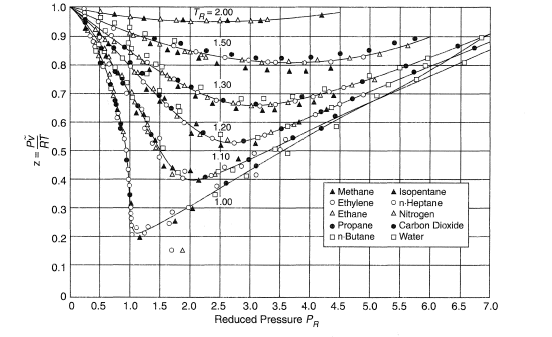
GAS LAW
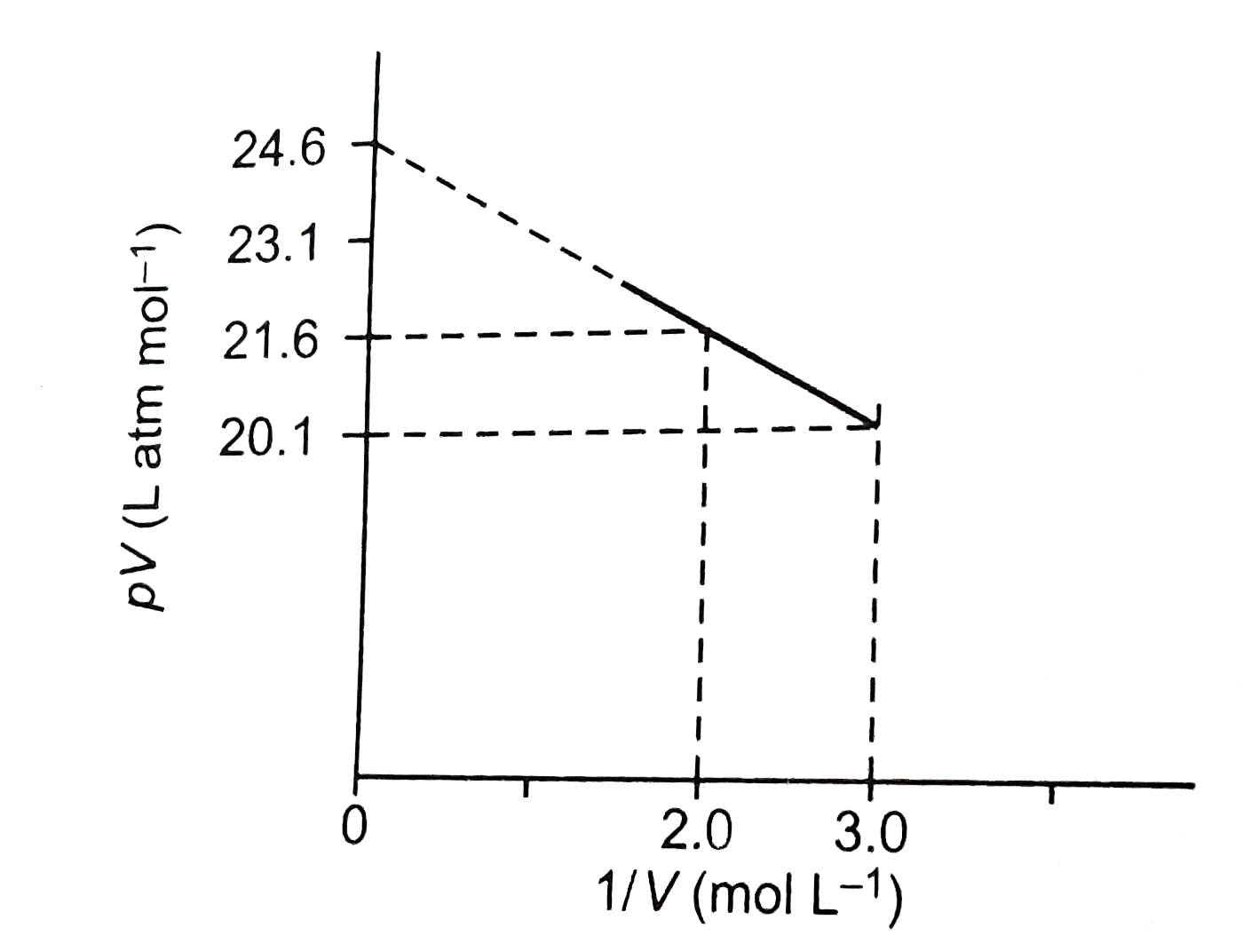
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12
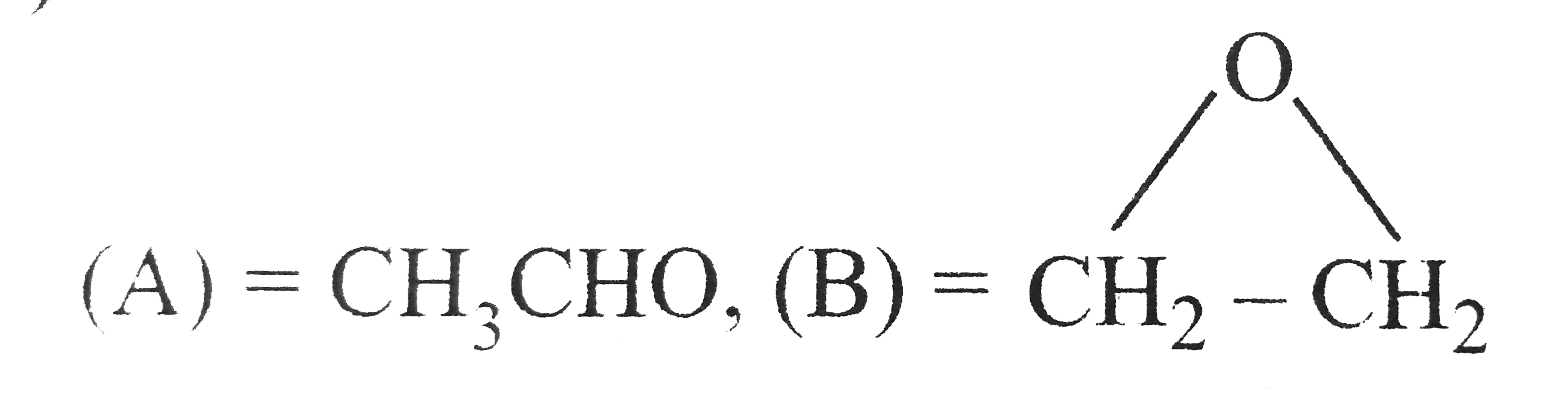
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions