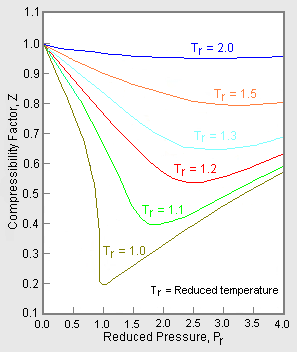
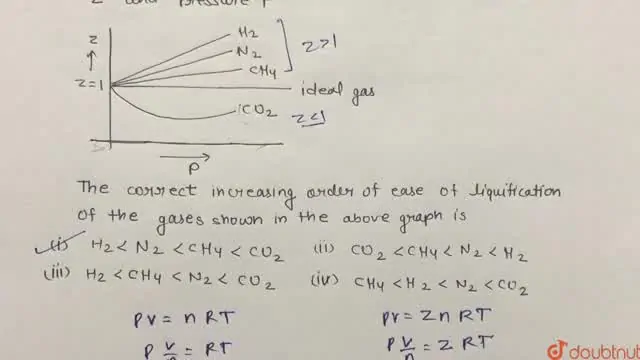
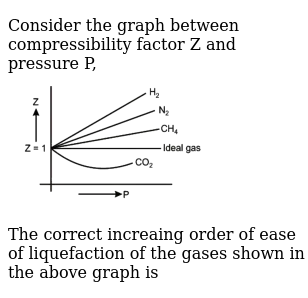
Consider the graph between compressibility factor Z and pressure P

By A Mystery Man Writer
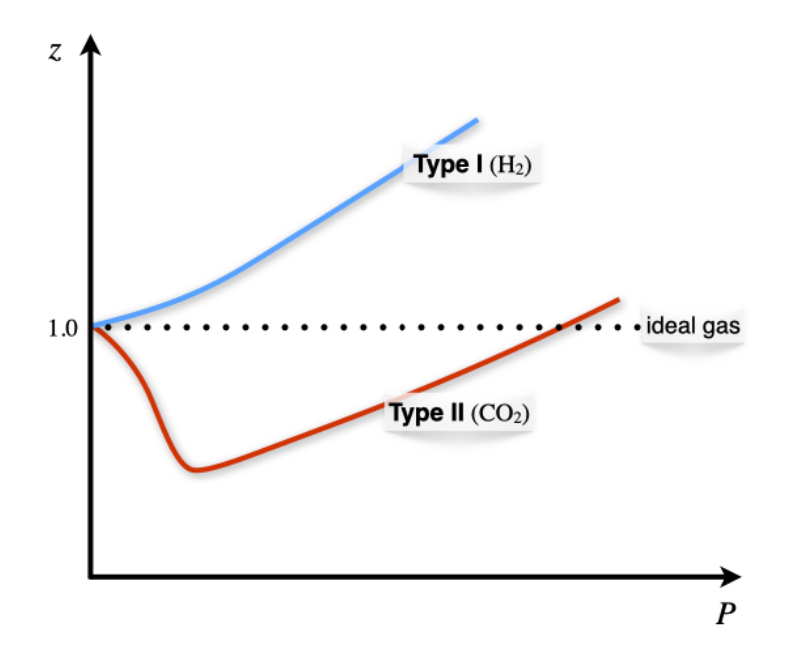
Z1 means force of attraction dominating ie a is considerable b can be negligible at low temperature and low pressure Lower is the value of Z easier is the process of liquification
The compressibility factor is actually a factor that corrects the actual value of the gas versus the ideal gas. Let us learn and understand this concept.
Watch this video to understand the behaviour of real gases with the help of the compressibility factor. This is an important topic for JEE main.
What is the compressibility factor, and how does it vary with an increase in temperature and pressure? Watch this video to get the answer. This is an importa
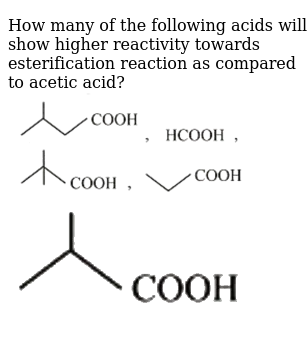
How many of the following acids will show higher reactivity towards es
11.3: Critical Phenomena - Chemistry LibreTexts

A graph Z vs P is plotted N_2 gas different temperatureThe correct relationship between temperatures
Consider the graph between compressibility factor Z and pressure P
Consider the graph between compressibility factor Z and pressure P

The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following

Consider the graph between compressibility factor Z and pressure P. The correct increasing order of ease of liquefaction of the gases shown in the above graph is
What is the significance of the curve part in Z vs. P graph of compressibility of a gas? - Quora
Telugu] The variation of compressibility factor (Z) with pressure (p
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
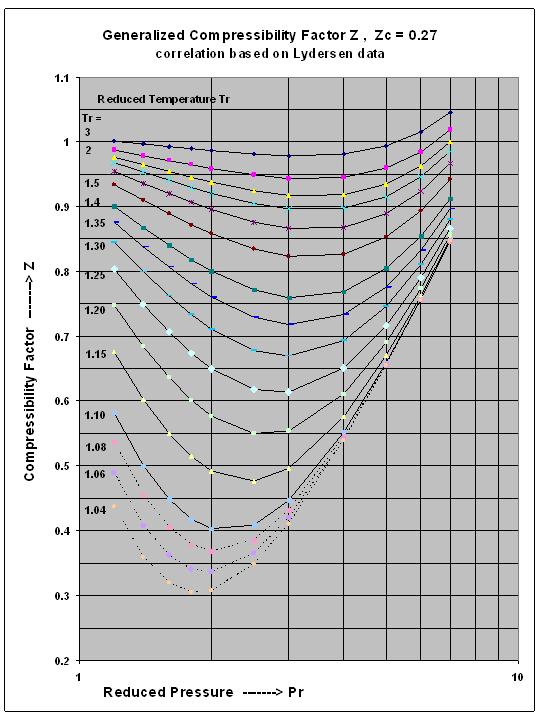
Super-critical Fluid Compressibility Factor Z , for Intermediate Reduced Pressure Range, a new correlation for excel spreadsheets
- Activar una tarjeta Pandemic-EBT para acceder a los beneficios de alimentos

- BOOB TAPE WITH 10 PAIRS NIPPLE COVER COTTON WIDE THIN BREAST TAPE - WOMEN'S & GIRL'S BREAST

- Women Unitard Dance Bodysuit High Neck Dancer Leotard Full Body Suits One Piece Long Sleeve Turtleneck Jumpsuit

- Calvin Klein Women's Modern Cotton Skinny Strap Bralette Grey, XL

- 5Pcs Bulk Items Wholesale Ladies Dress Summer Sexy High Waist