24. Assertion :In B2H6, the terminal B H bonds are shorter, than
By A Mystery Man Writer
24. Assertion :In B2H6, the terminal B H bonds are shorter, than the B H bridge bonds Reason: The terminal B H bond order is greater than that of the B H bridge bond
24- Assertion-In B2H6- the terminal B-H bonds are shorter- than the B-H bridge bonds Reason- The terminal B-H bond order is greater than that of the B-H bridge bond
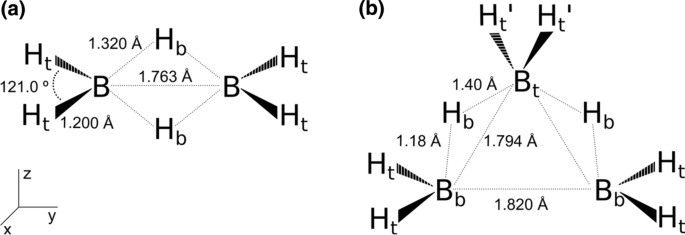
Three-center two-electron bonds in the boranes B2H6 and B3H8− from the quantum interference perspective

ChemicalBondingBYPMS PDF
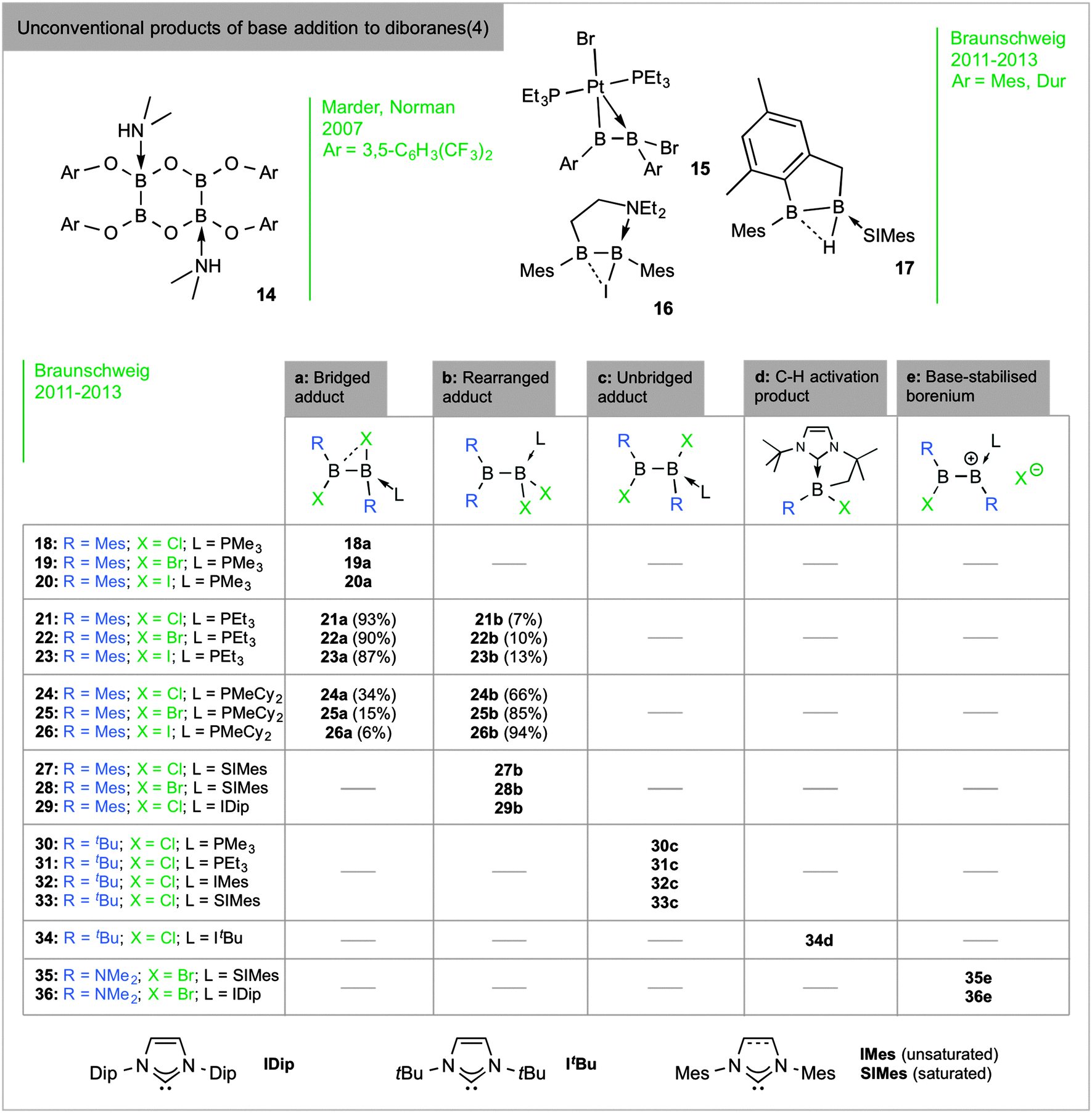
sp 2 –sp 3 diboranes: astounding structural variability and mild sources of nucleophilic boron for organic synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C5CC02316E

Inorganic Chemistry Chapter-1-8 PDF, PDF, Ion

B2H6 bonding

1 M3 2 Chemical Bonding, PDF, Ionic Bonding

All the B - H bonds in B_2H_6 are equivalent.truefalse
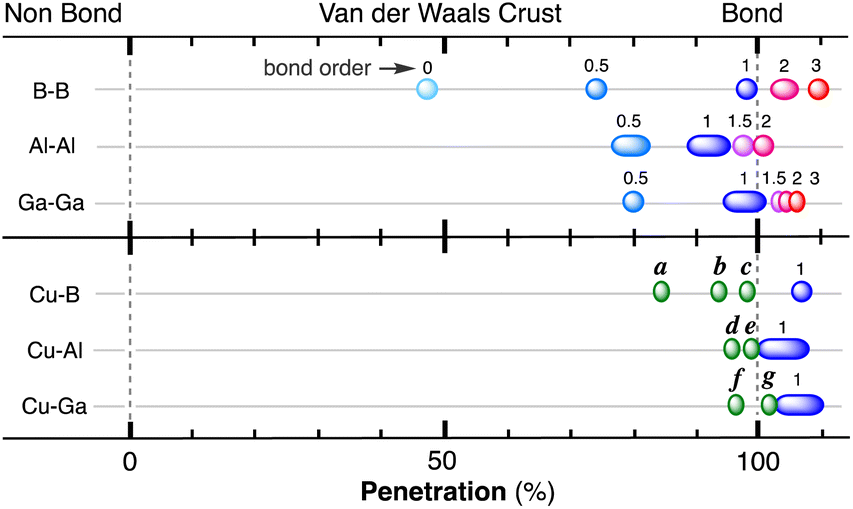
The borderless world of chemical bonding across the van der Waals crust and the valence region - Chemical Science (RSC Publishing) DOI:10.1039/D3SC02238B

Solved] Which of the following statement is not correct about dibora
sp 2 –sp 3 diboranes: astounding structural variability and mild sources of nucleophilic boron for organic synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C5CC02316E
- Dome lid
- Plus Size - Full Length Signature Waist Fleece-Lined Pocket
- No Boundaries Bra Size Small for Sale in Huntingdon, PA - OfferUp

- Singapore Bras Basah Road,Raffles Boulevard,Asian teen teens

- 70s Disco Pants for Mens Stage Performance Costumes Bell Bottom

- Vintage Hagen Renaker Cottontail Rabbit Bunny Baby Miniature Ceramic - Ruby Lane

- Eden Rashguard - Pepla

- Disney, Intimates & Sleepwear

- Women Fleece Hooded Bathrobe Plush Soft Long Robe Fluffy Warm Bath Robes Sherpa Shaggy Housecoat Pajama with Pocket : : Clothing, Shoes & Accessories

- Warners Womens Plus Size Signature Cushioned Support and Comfort Underwire Unlined Full-Coverage Bra 35002a







