a) Atomic structure of I–42d FeMg2O4 and Fe and Mg coordination

By A Mystery Man Writer

PDF) Synthesis and superstructural characterization of Fe1.89Mo4.11O7

Renhai Wang's research works GuangDong University of Technology

Fang YIMEI, PhD Student, Xiamen University, Xiamen, XMU, Department of Physics

Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why? - Zigya

Explain structure of ferrocyanide ion? Explain the coordinating bonding between Fe and cyanide ion? How many cyanide ion form covalent and coordinate bond? Explain full structure with detail - 53ksb3aa

Zijing LIN University of Science and Technology of China, Hefei

Zijing LIN, University of Science and Technology of China, Hefei, USTC, Department of Physics

Shunqing WU, Professor, PhD, Xiamen University, Xiamen

Explain structure of ferrocyanide ion? Explain the coordinating bonding between Fe and cyanide ion? How many cyanide ion form covalent and coordinate bond? Explain full structure with detail - 53ksb3aa

Feng ZHENG, Xiamen University, Xiamen, XMU
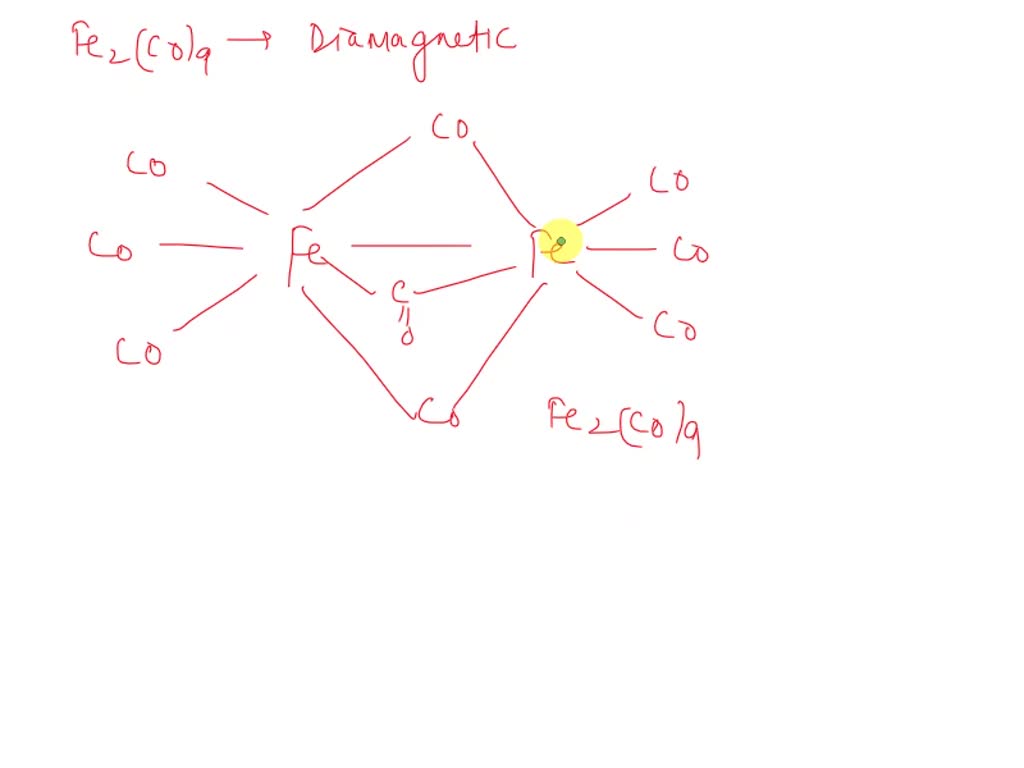
SOLVED: Fe2(CO)9 is diamagnetic. Which of the following reasons is correct? A. Presence of one CO as a bridge group B. Presence of monodentate ligand C. Metal-metal (Fe-Fe) bond in the molecule

Structural transition and re-emergence of iron's total electron spin in (Mg, Fe)O at ultrahigh pressure









