42g of N₂ react with excess of O₂ to produce NO. Amount of NO

By A Mystery Man Writer
Share your videos with friends, family, and the world
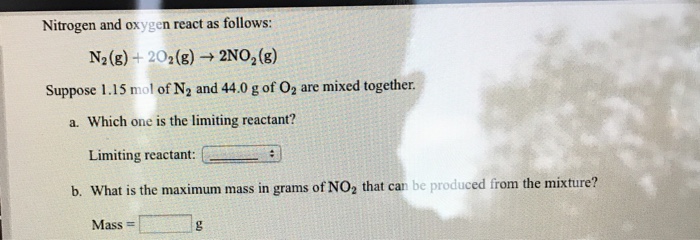
Solved Nitrogen and oxygen react as follows: N2(g)-202(g) →

Empirical formula of a hydrocarbon having 80% C and 20% of hydrogen is a.CH b.CH3 c.CH2 d.CH4 MDCAT

Limiting Reaction Calculations Practice Flashcards
UMAIR KHAN ACADEMY

Limiting Reaction Calculations Practice Flashcards

Answered: LIMITING REAGENTS TO2/S21 Based on the…

Solved Consider the reaction N2(g) +202(g) + 2NO2(9). The
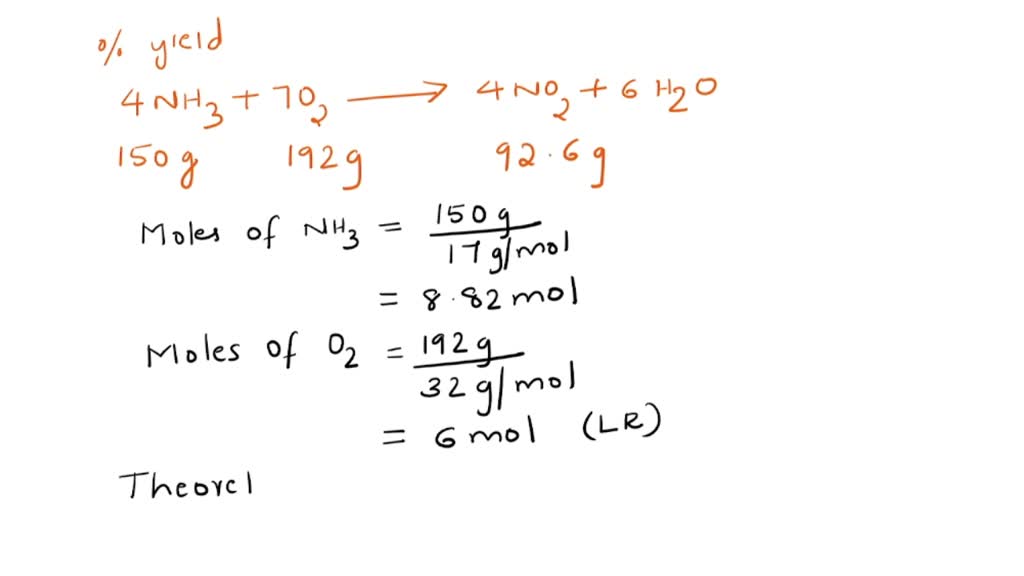
SOLVED: The combustion of ammonia in the presence of oxygen yields NO2 and H2O: 4 NH3 (g) + 7 O2 (g) → 4 NO2 (g) + 6 H2O (g) The combustion 150.

Chemistry in Daily Life Homework Help, Questions with Solutions - Kunduz

Mole Concept PDF, PDF, Mole (Unit)

UMAIR KHAN ACADEMY
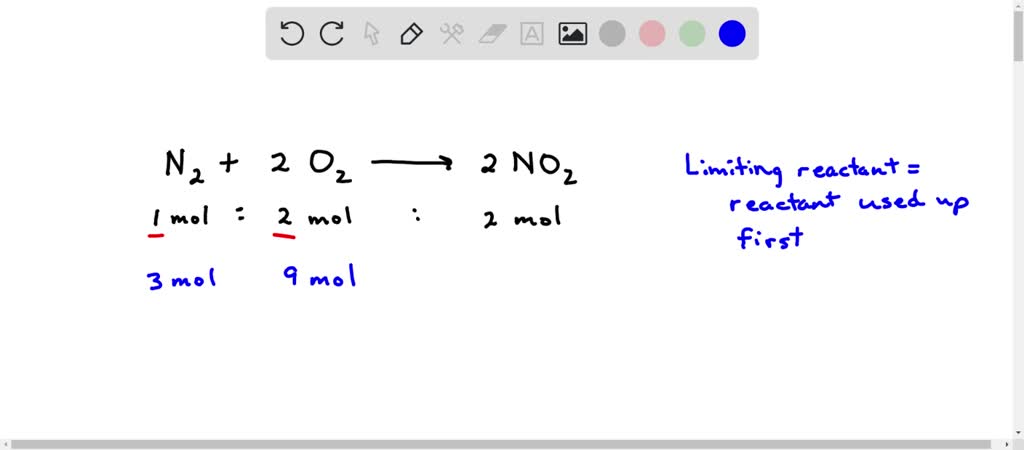
SOLVED: N2(g) + 2O2(g) â†' 2NO2(g). What is the limiting reactant when 3 moles of N2 and 9 moles of O2 react, and how much of the excess reactant remains after the
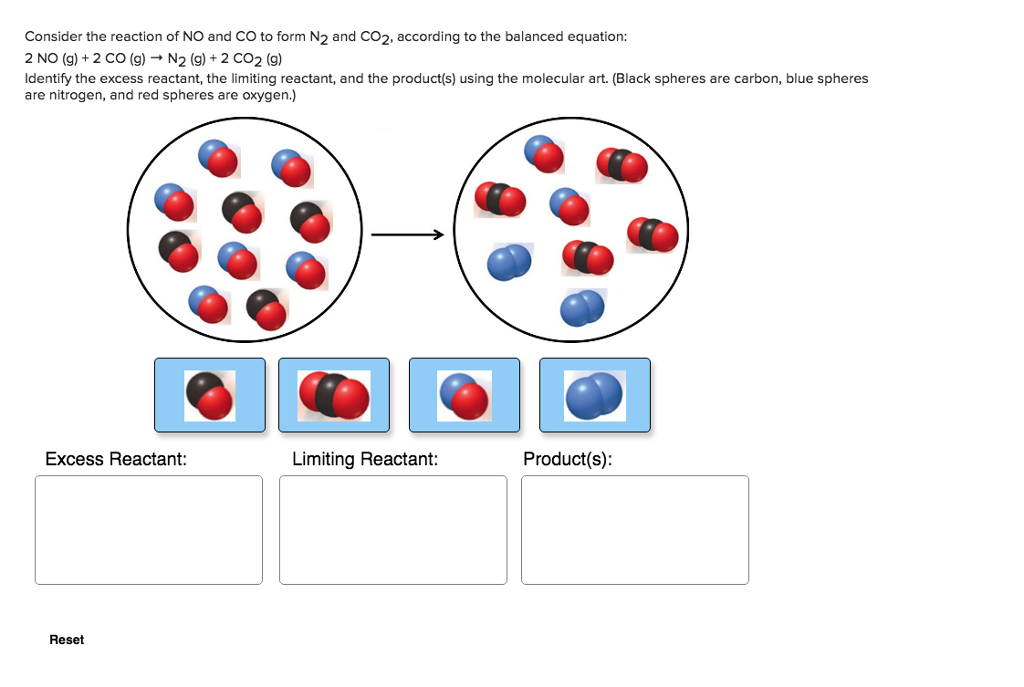
Solved Consider the reaction of NO and CO to form N2 and
- NESTLE LION CHOC. BR. WAFER C/ FLOCOS ARROZ E CARAMELO 42G
- Bolinho de Baunilha com Recheio e Cobertura Sabor Chocolate Ana

- FIMO Kids, white, Nr. 0, 42g (1.5oz), oven-hardening polymer clay

- FD-42G PANASONIC - Sensor: fiber-optic, Range: 0÷200mm; IP40; -55÷80°C; Len: 2m

- FD-42G Panasonic, SUNX Reflective-Type Fiber Sensor

- Ultra Stretch High Rise Denim Leggings Pants

- Legging de Poliamida com Bolso Mescla - Livre e Leve

- Maidenform Womens Pure Comfort Boy Brief Dm224c : : Clothing, Shoes & Accessories

- Gotoly Women's Compression Tank Tops Triple Tummy Control Workout Tops Shapewear Camisole Cami Shaper with Zipper Body Shaper(Black, Small) at Women's Clothing store

- Morph: Flyout Mobile Menu Plugin for WordPress, WP Plugins ft. mobile & menu - Envato Elements

