PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis.

By A Mystery Man Writer
It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains in white-tailed deer. A variety of aberrant erythrocyte forms have been related to seven adult and two fetal hemoglobins in white-tailed deer. While sickling of the erythrocyte was not associated with a single hemoglobin type, it was precluded by hemoglobin V or VII, even when in combination with other hemoglobin types normally associated with sickling. The subunit basis of the hemoglobin polymorphism was presented. Two kinds of α subunits, six kinds of β subunits and one γ subunit were related to the whole hemoglobin molecule. The heterogeneity of the deer hemoglobins was based upon a variety of combinations of these numerous polypeptide chains. It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains.
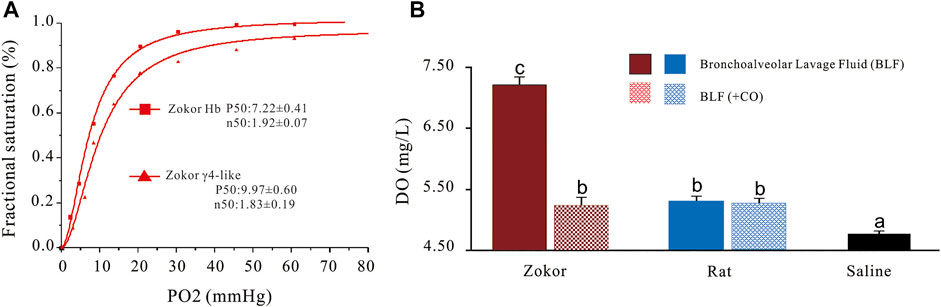
Frontiers A New Homotetramer Hemoglobin in the Pulmonary Surfactant of Plateau Zokors (Myospalax Baileyi)

PDF) Parallel evolution in the major hemoglobin genes of eight species of Andean waterfowl

Hematology SpringerLink

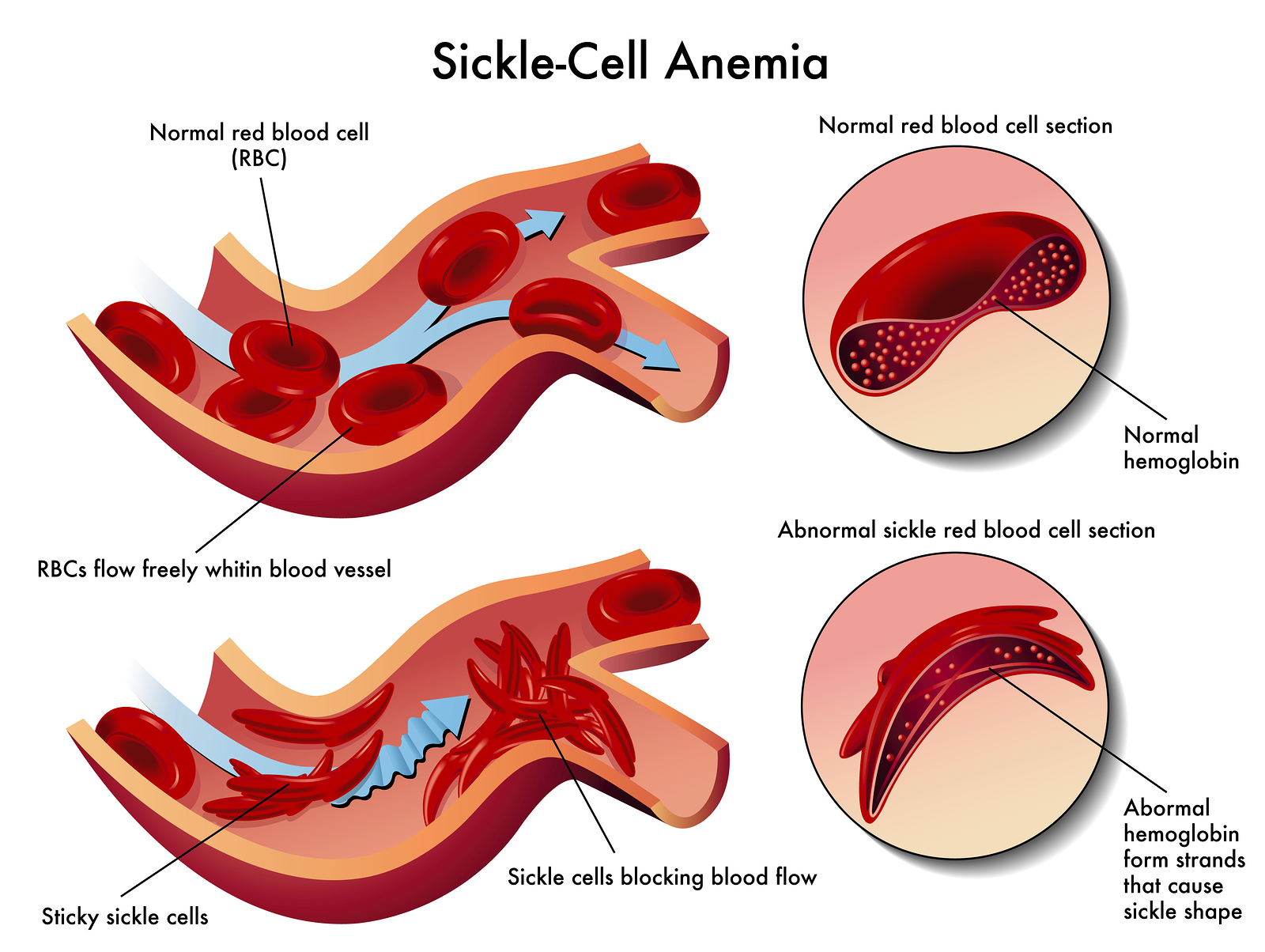
Ultrastructure of Sickled Deer Erythrocytes. I. The Typical Crescent and Holly Leaf Forms - ScienceDirect

Animals, Free Full-Text

Lubert Stryer - Biochemistry.pdf

Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? - Abstract - Europe PMC
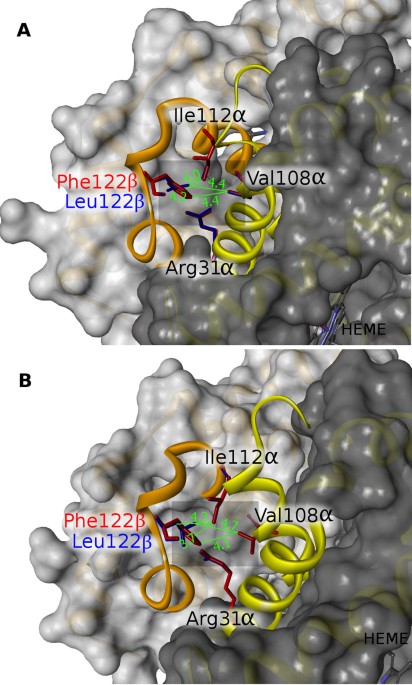
The conserved Phe GH5 of importance for hemoglobin intersubunit contact is mutated in gadoid fish, BMC Ecology and Evolution
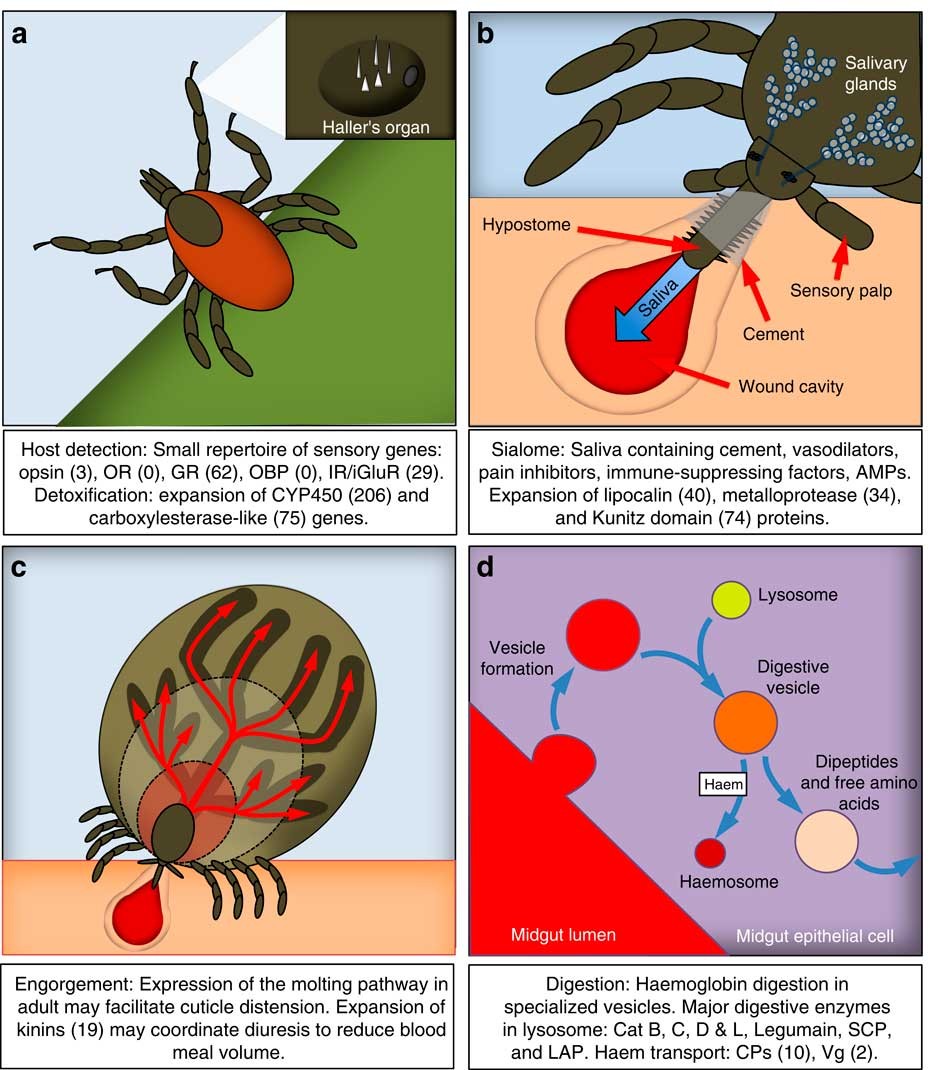
Genomic insights into the Ixodes scapularis tick vector of Lyme disease

High-altitude deer mouse hypoxia-inducible factor-2α shows defective interaction with CREB-binding protein - ScienceDirect
- Laboratory Evaluation of Sickle Cell Disease in the ED — Taming
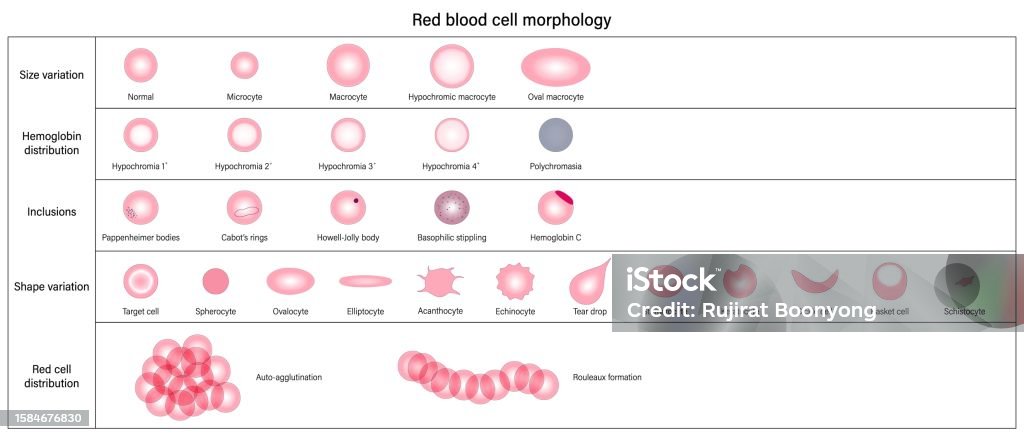
- Vetores de Morfologia Das Hemácias Variação De Tamanho Hemoglobina
- HEMO Shapewear Women's Slimming Shapewear for Women Bummach

- HEMO Shapewear Women's Slimming Shapewear for Women Bummach Control Body Shaper Open Bust Bodysuit Compression Firm Bummach Control Shaper Corsage (Colour: Black, Size: Medium) : : Fashion

- HEMOPLT Pack of 6 Self Watering Planters, 5/6.8 Inch Simulated Metallic Plant Pots, Thickness of 0.15 Sturdy Flower Pots, Ideal Birthday (Champagne Gold, Black Silver, Rose Gold) Plastic





