Sunday, Sept 29 2024
physical chemistry - Is the compressibility factor smaller or

By A Mystery Man Writer
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Objectives_template

Mass & Volume Flow Rate, Overview & Equation - Lesson
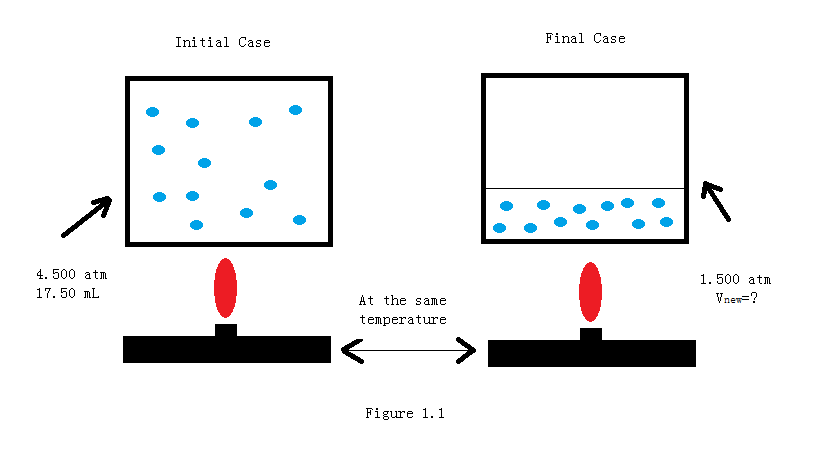
Gas Laws - Overview - Chemistry LibreTexts

Compressibility Factor Charts - Wolfram Demonstrations Project
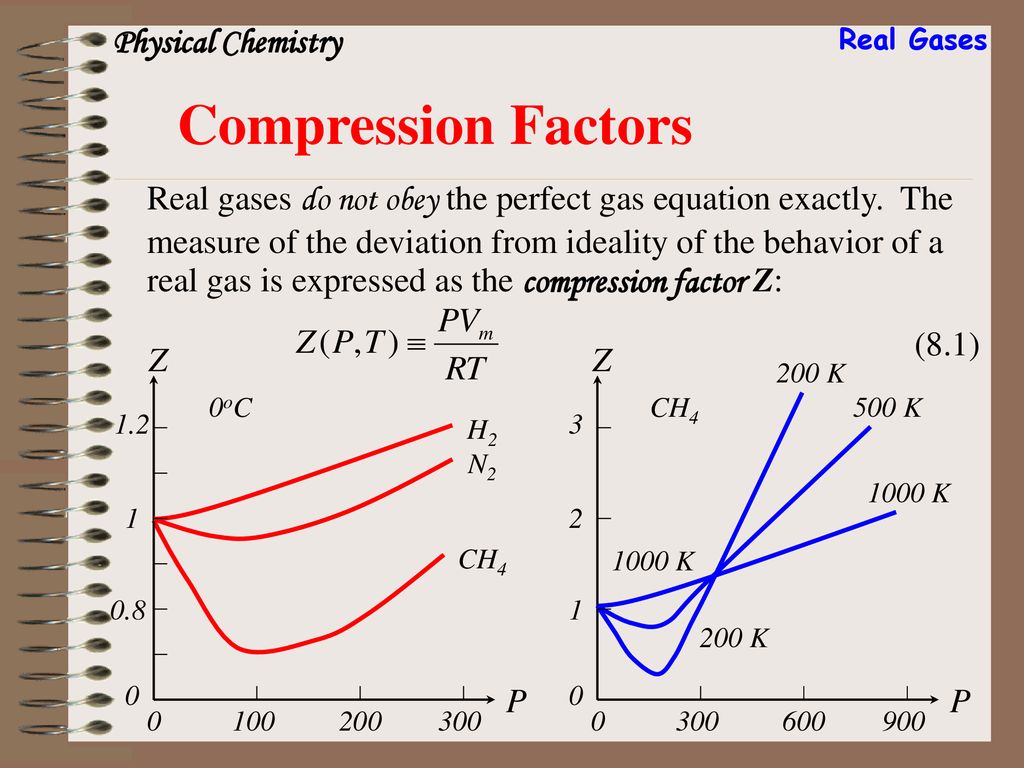
Chapter 8 Real Gases. - ppt download

Compressibility factor - Wikipedia

Gas compressibility factor Z: Ideal gas vs Real gas
The periodic table and the physics that drives it

Physics Chemistry Mathematics Q.5 Single Choice Deviation of a

Gas compressibility factor Z: Ideal gas vs Real gas
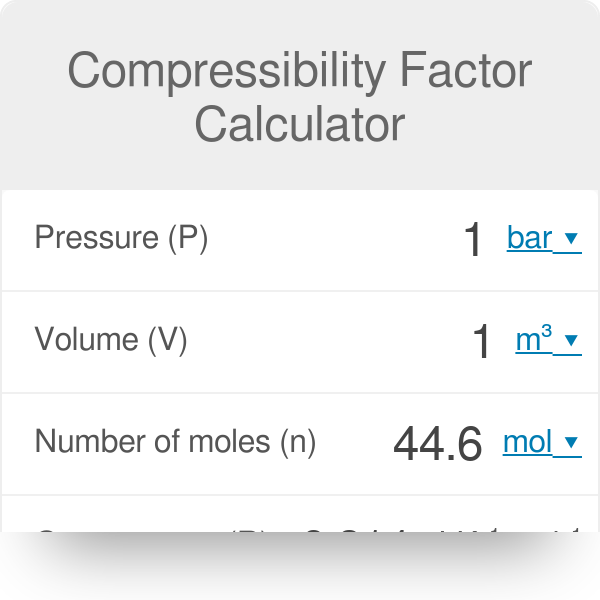
Compressibility Factor Calculator
Related searches
- Compressibility factor (gases) - Knowino
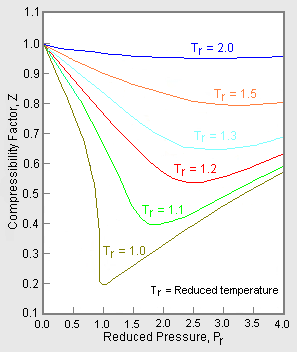
- PDF] Compressibility Chart for Hydrogen and Inert Gases

- Write the expression for the compressibility factor (Z) for one

- Compressibility factor Z for sub-critical pressures in a 'one-cell' formula for excel spreadsheets

- physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

©2016-2024, reintegratieinactie.nl, Inc. or its affiliates




