Health-related quality of life and quality-adjusted progression
By A Mystery Man Writer
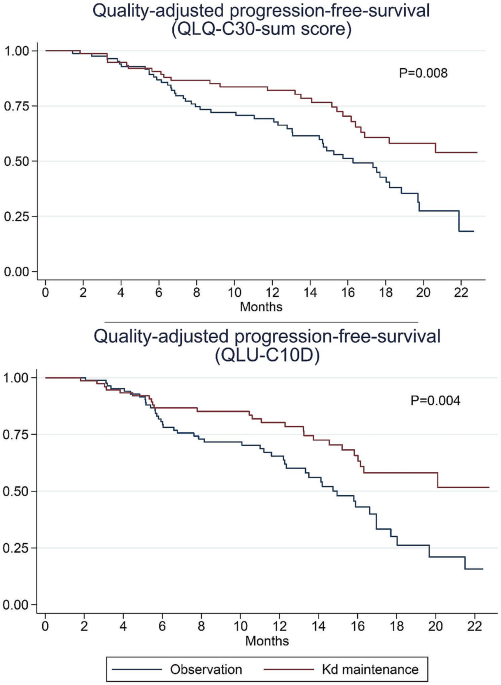
Background Decisions regarding maintenance therapy in patients with multiple myeloma should be based on both treatment efficacy and health-related quality of life (HRQL) consequences. In the CARFI trial, patients with first relapse of multiple myeloma underwent salvage autologous stem cell transplantation (salvage ASCT) before randomization to carfilzomib-dexamethasone maintenance therapy (Kd) or observation. The primary clinical endpoint was time to progression, which was extended by 8 months by Kd. The aim of this paper is to present the all HRQL endpoints of the CARFI trial including the HRQL effect of Kd maintenance therapy relative to observation. The primary HRQL endpoint was assessed by EORTC QLQ-C30 Summary score (QLQ-C30-sum) at 8 months follow-up. A key secondary HRQL endpoint was quality-adjusted progression-free-survival (QAPFS). Methods HRQL was assessed with EORTC QLQ-C30, EORTC QLQ-MY20 and FACT/GOG-Ntx at randomization and every second month during follow-up. HRQL data were analyzed with linear mixed effect models until 8 months follow-up. QAPFS per individual was calculated by multiplying progression-free survival (PFS) by two quality-adjustment metrics, the QLQ-C30-sum and EORTC Quality of Life Utility Measure-Core 10 dimensions (QLU-C10D). The QAPFS per treatment group was estimated with the Kaplan-Meier method. P < 0.05 was used for statistical significance, and a between-group minimal important difference of 10 points was interpreted as clinically relevant for the QLQ-C30-sum. Results 168 patients were randomized. HRQL questionnaire compliance was 93%. For the QLQ-C30-sum, the difference of 4.62 points (95% confidence interval (CI) -8.9: -0.4, p = 0.032) was not clinically relevant. PFS was 19.3 months for the Kd maintenance group and 16.8 months for the observation group; difference = 2.5 months (95% CI 0.5; 4.5). QAPFS based on the QLQ-C30-sum for the Kd maintenance group was 18.0 months (95% CI 16.4; 19.6) and for the observation group 15.0 months (95% CI 13.5; 16.5); difference = 3.0 months (95% CI 0.8–5.3). QAPFS based on the QLU-C10D for the Kd maintenance group was 17.5 months (95% CI 15.9; 19.2) and 14.0 months (95% CI 12.4; 15.5) for the observation group; difference = 3.5 months (95% CI 1.1–5.9). Conclusions Kd maintenance therapy after salvage ASCT did not adversely affect overall HRQL, but adjustment for HRQL reduced the PFS compared to unadjusted PFS. PFS of maintenance therapy should be quality-adjusted to balance the benefits and HRQL impact.

Joint collaborations. Download Scientific Diagram

Impact of post-transplantation maintenance therapy on health-related quality of life in patients with multiple myeloma: data from the Connect® MM Registry
:max_bytes(150000):strip_icc()/Economic-Growth-FINAL-56d44a93534b46dab5793b2fec7b965e.jpg)
What Is Economic Growth and How Is It Measured?
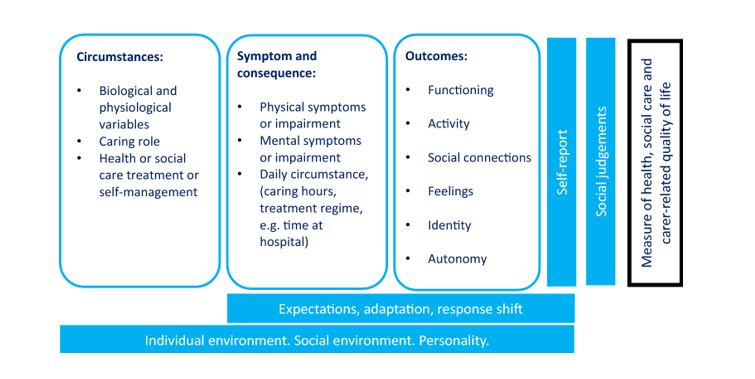
What is the best approach to adopt for identifying the domains for a new measure of health, social care and carer-related quality of life to measure quality-adjusted life years? Application to the

What Are Quality-adjusted Life Years (QALY)?

PDF) Valuing health-related quality of life among the Indian population: a protocol for the Development of an EQ-5D Value set for India using an Extended design (DEVINE) Study

Impact of post-transplantation maintenance therapy on health-related quality of life in patients with multiple myeloma: data from the Connect® MM Registry
Quality-adjusted life year - Wikipedia
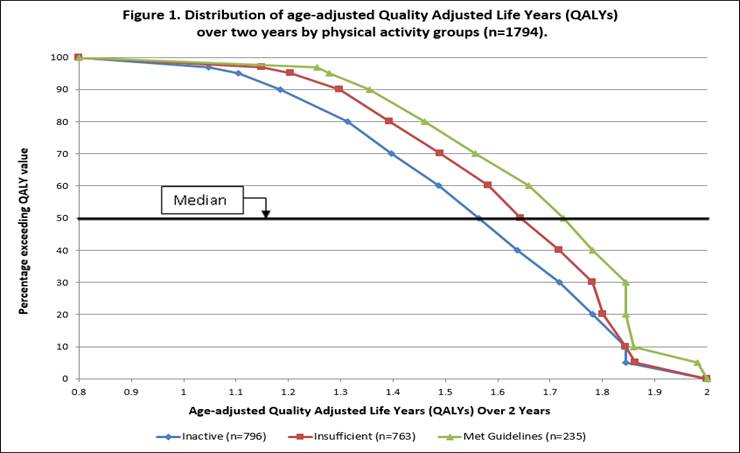
Relationship of Meeting Physical Activity Guidelines and Quality Adjusted Life Years - ACR Meeting Abstracts

The effect of comorbidity on health-related quality of life for injury patients in the first year following injury: comparison of three comorbidity adjustment approaches
:max_bytes(150000):strip_icc()/structural-adjustment.asp-final-686ebe7132e7455e985b2cdfb85cf56d.png)
What Are Structural Adjustment Programs (SAPs)?

Health-related quality of life and quality-adjusted progression free survival for carfilzomib and dexamethasone maintenance following salvage autologous stem-cell transplantation in patients with multiple myeloma: a randomized phase 2 trial by the Nordic

Association between racial discrimination and health-related

Understanding the ADDIE Model: All You Need to Know + Template - AIHR

PDF) Health-related quality of life in transplant ineligible newly diagnosed multiple myeloma patients treated with either thalidomide or lenalidomide-based regimen until progression: A prospective, open-label, multicenter, randomized, phase 3 study
- The CORE-bots have arrived - Therapy Meets Numbers

- Full article: The adaptation of a community-based suicide prevention intervention during the COVID19 pandemic: a mixed method study

- 📝🇨🇦The PTE Core is the third option for an English language test that candidates can take for their permanent residency application. The ot…
- Phase 10 Score Sheets: 1500 Large Print Score Pads for Scorekeeping - Phase Ten Score Cards: Kim, Keith: 9798499829578: : Books

- Mixed methods feasibility and usability testing of a childhood
- Curvy Girls Pole - Green Teacup's Pole Collection Leggings for

- Panache Ana Plunge Bra - Core Colors – Filly Rose

- Ampro's Shine 'n Jam Hair Gel Is My New Favorite Hair Gel — Editor Review

- SPORTGEL COLD CREAM 100ML

- Bali Women's Lightly Lined Double Support Wire-Free Bra, White, 34B at Women's Clothing store: Bras

