The value of compression factor at the critical state of a vander
By A Mystery Man Writer
The value of compression factor at the critical state of a vander waals gas is
SOLVED: 1) Estimate/ Calculate the critical constants (pc, Vc, and Tc) for a gas molecule whose van der Waals parameters are a = 1.32 atm dm^6 mol^-2 and b = 0.0436 dm^3
6.3: Van der Waals and Other Gases - Physics LibreTexts

Compressibility factor - Wikipedia
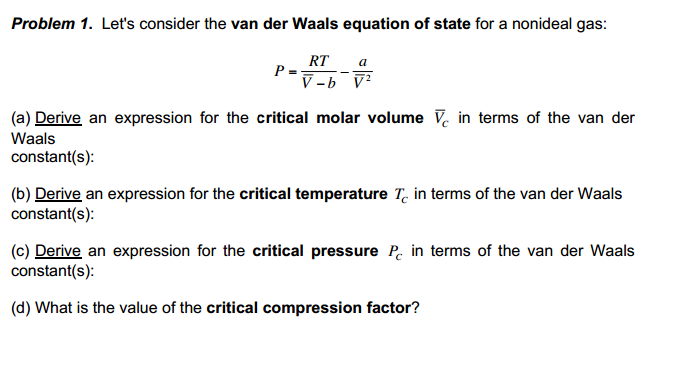
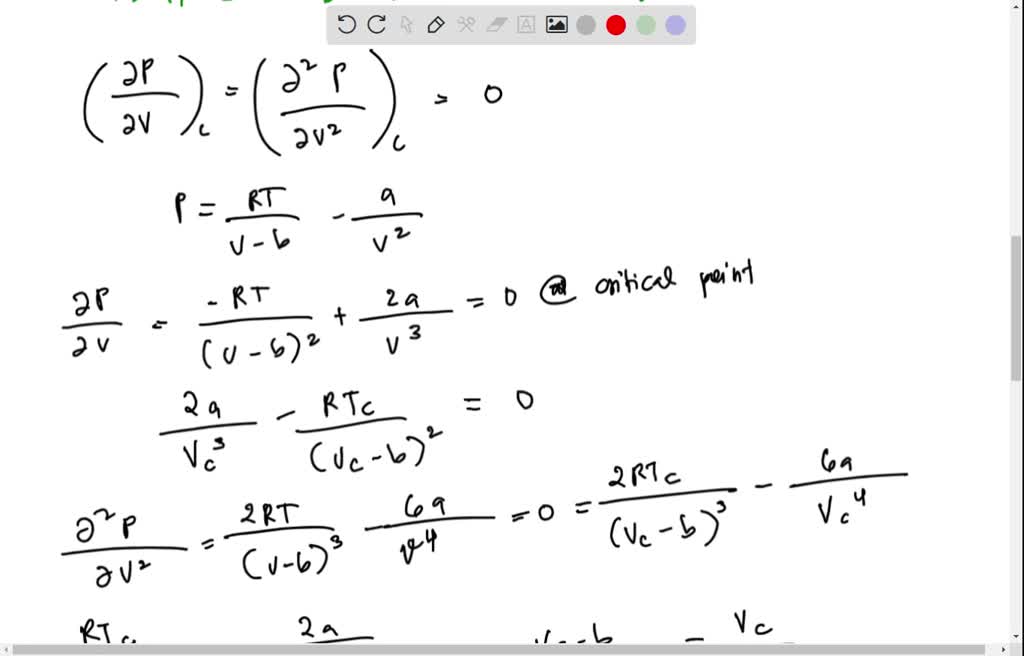
Solved Problem 1. Let's consider the van der Waals equation

What is the value of z (compressibility factor) for a vander waal gas at critical

At high pressure, the compressibility factor for one mole of van der w

The compressibility factor in terms of Pc, Vc and Tc is called Zc. Th

Real Gases and the van der Waals Equation Explained

Solved I need help on Problem 2: a,b,c,d,e,f. I'm stuck on

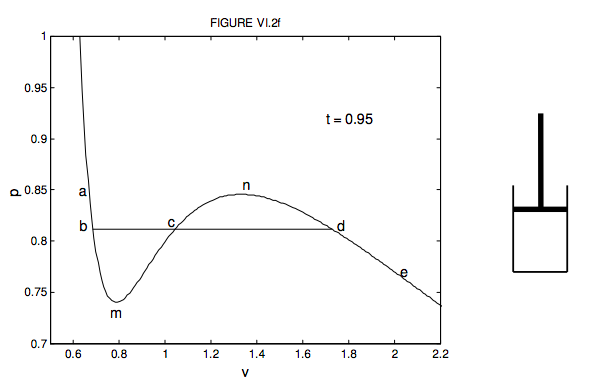
108. Which of following statement (s) is true 1 - Slope of

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

Compressibility factor - Wikipedia
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora

Critical constants and parameters of the PRSV equation for hydrogen
- Explain how the compression factor varies with pressure and

- Compressibility Factor Charts - Wolfram Demonstrations Project

- Gas Compressibility Factor Spreadsheet Calculator

- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
- 000559 Calculation of Compressibility Factor from Redlich-Kwong

- Exotic Goth Accessories Fetish Lingerie Set Harness For Busty Women Plus Size Underwear Cage Chest Festival Rave Wear Garter - Garters - AliExpress

- Spanx Green Camo Look At Me Now High Waisted Seamless Leggings

- Men Stretchy Ripped Skinny Jeans Destroyed Taped Slim Fit Denim

- Buy Silvertraq Women's Aura Leggings

- Three Members of No. 17 Warrior Women's Wrestling Go Unbeaten at Simpson - Corban University Athletics
