Compression of a gas due to external pressure and the

By A Mystery Man Writer

One mole of an ideal gas is compressed from 500 cm^(3) against a

The work done in adiabatic compression of `2` mole of an ideal monoatomic gas by constant
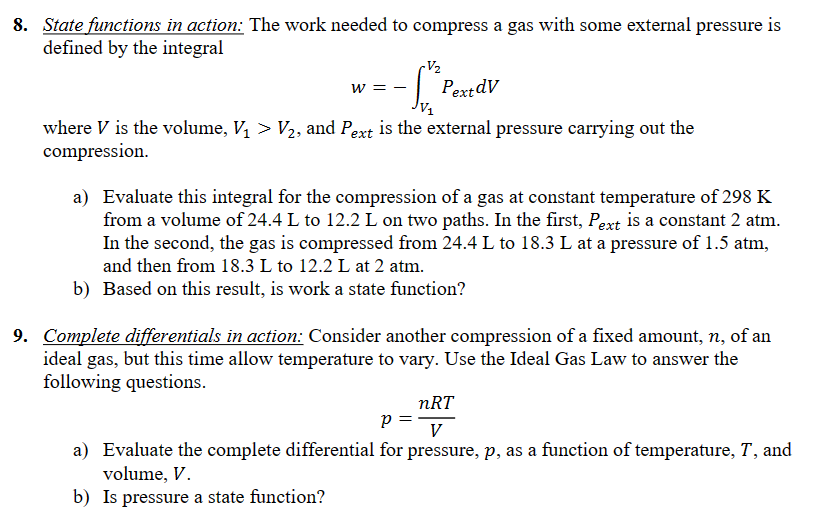
Solved State functions in action: The work needed to

Compression, Pressure, Force & Volume

Entropy Contributions by: - ppt download

Cristian MERINO RUBILAR, Professor (Assistant)
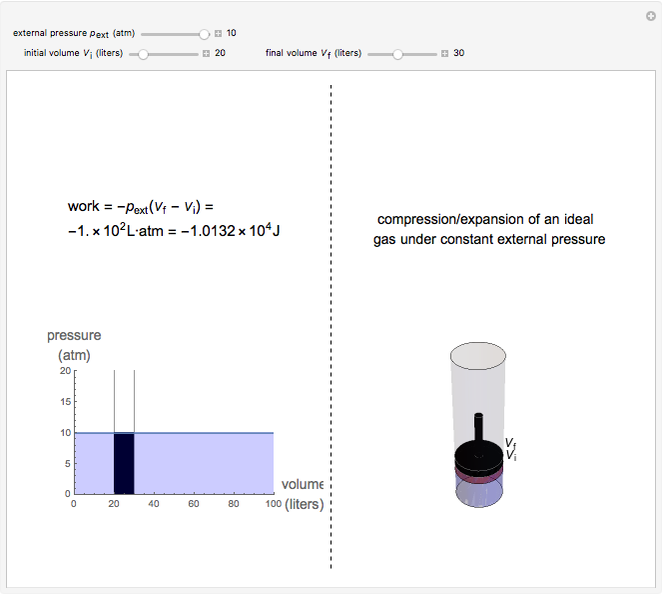
Isobaric Compression and Expansion of an Ideal Gas - Wolfram Demonstrations Project

SOLVED: A gas is compressed from an initial volume of 5.55 L to a final volume of 1.24 L by an external pressure of 1.00 atm . During the compression the gas

thermodynamics - Immediate pressure change after compressing a gas - Physics Stack Exchange

1 (3) 248.5 K Pere R2- (4) 200 K La The work done in adiabatic compression of 2 mole of an ideal monoatomic gas by constant extemal pressure of 2 atm

The work done in adiabatic compression of `2` mole of an ideal monoatomic gas by constant

12.2 First law of Thermodynamics: Thermal Energy and Work

Waldo QUIROZ, Professor (Full), PhD Chemistry
Two moles of an ideal gas is compressed isothermally and reversibly from a volume 2L to 0.5L at initial pressure of 1 atm . the work done by gas i
- Dorman High Pressure Compression Union Rated For 5000 PSI 3/16 In. 800-202 - Advance Auto Parts

- VEVOR Compression Tester Adapter Kit 9 PCS Automotive Engine Cylinder Leak Down Compression Test Pressure Gauge 0-300 psi. QCQGYLCSYQY0XJV75V0 - The Home Depot

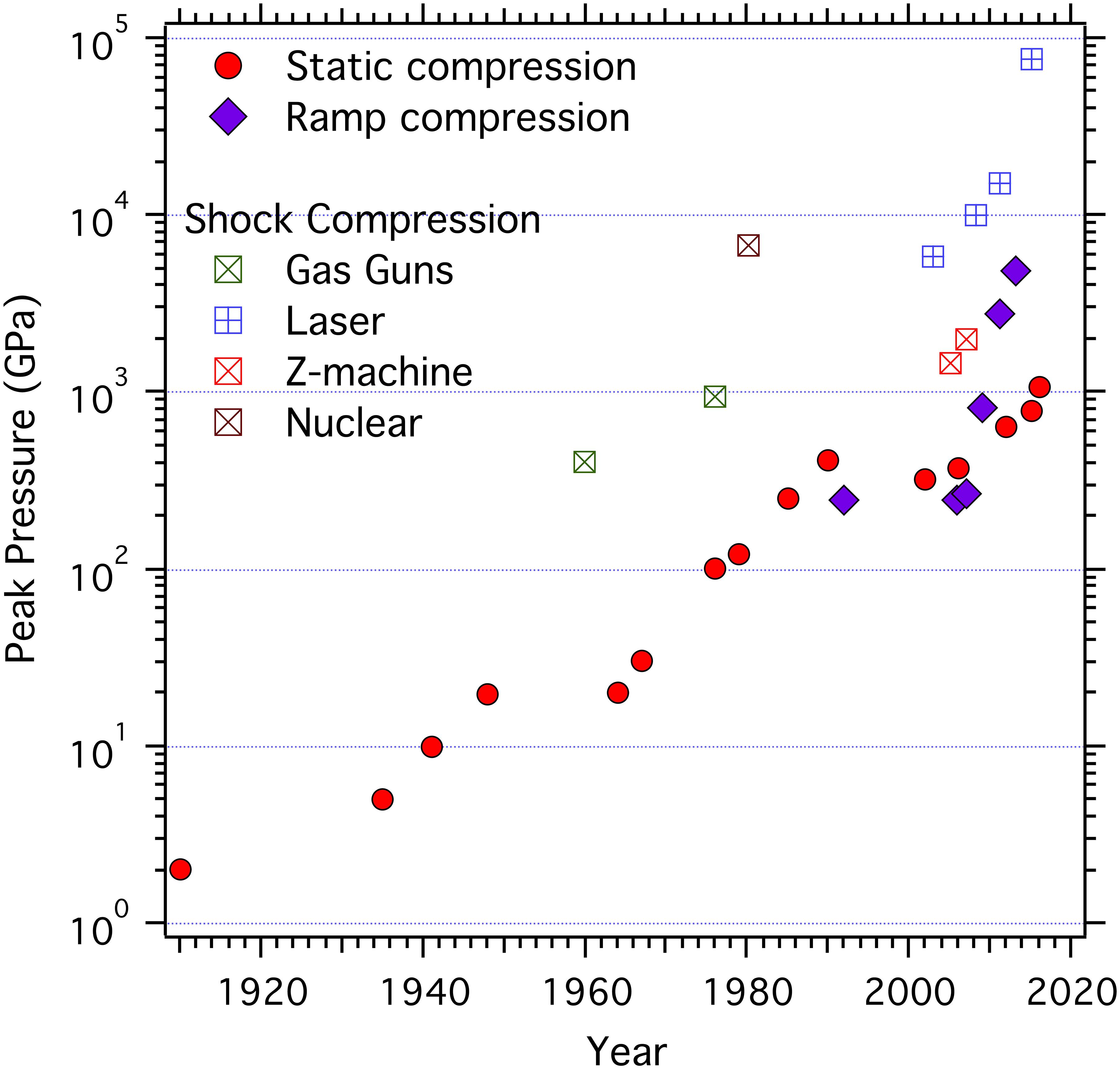
- Frontiers Ultra-High Pressure Dynamic Compression of Geological Materials
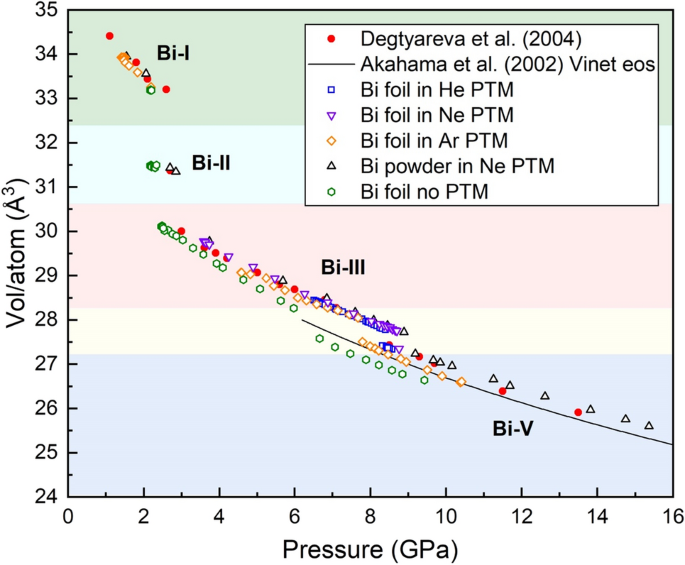
- Compression-rate dependence of pressure-induced phase transitions
- Biomechanics, Free Full-Text
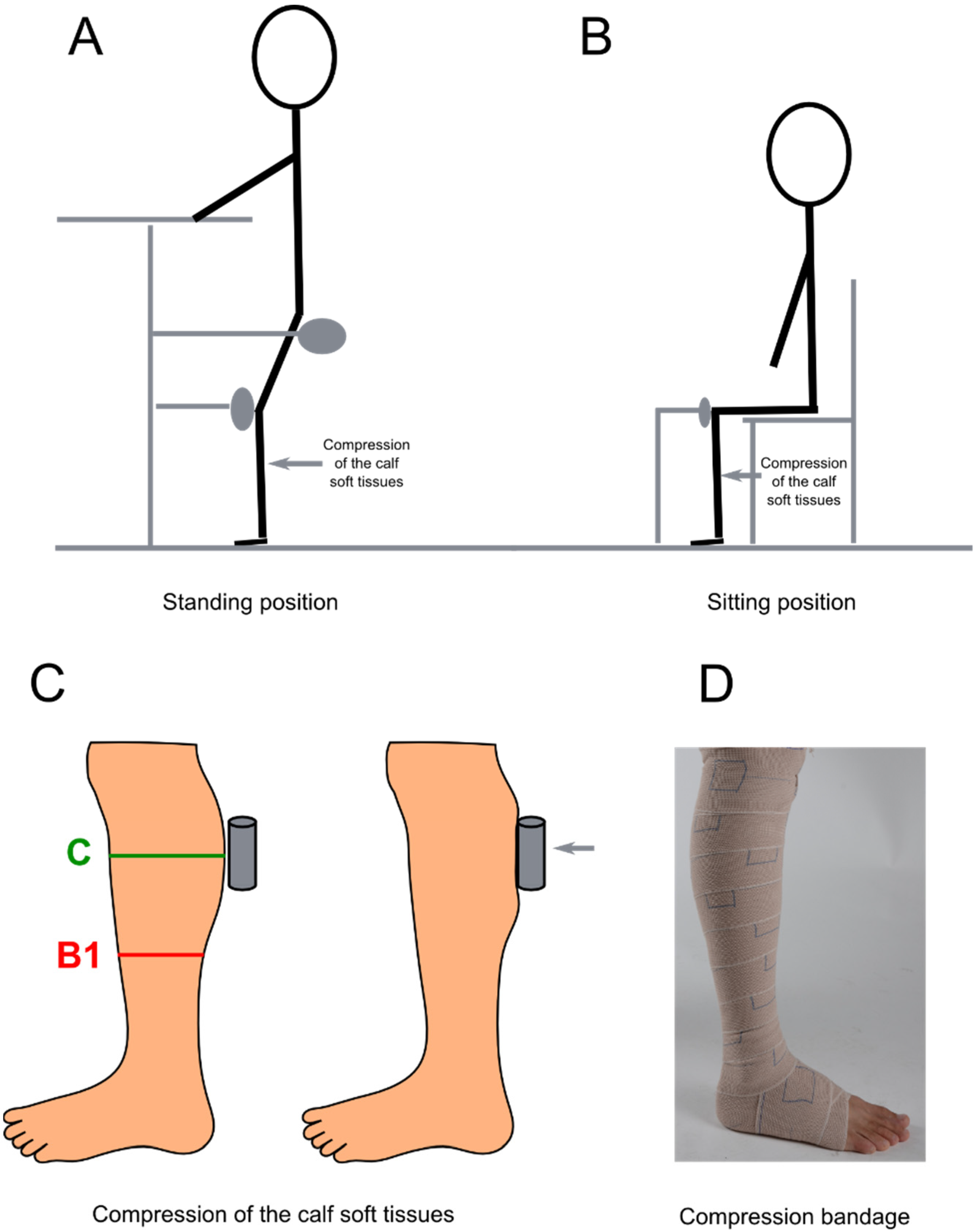
- 3 Ways to Choose and Wear a Protective Cup for Sports - wikiHow

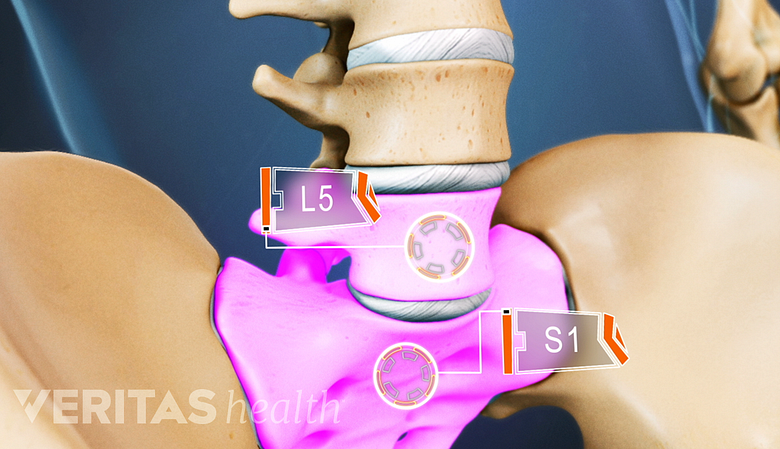
- Todo sobre el segmento vertebral L5-S1 (articulación lumbosacra)
- Fila + FILA x TALA SKINLUXE Leggings

- KORALHY Tennis Polo Women, Ladies Golf Shirts Short Sleeve Moisture Wicking Performance Cool Quick-Dry Sweat-Wicking Sport Workout Running Shirts Blue Large

- Womens FABLETICS Red Stretch Seamless High Rise 24-7 Skinny Pants
