Calculate the number of molecules of CO_2 present in 4.4 g of it.

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:calculate the number of molecules of co2 present in 44 g of it
Click here👆to get an answer to your question ✍️ Calculate the number of molecules of CO-2 present in 4-4 g of it
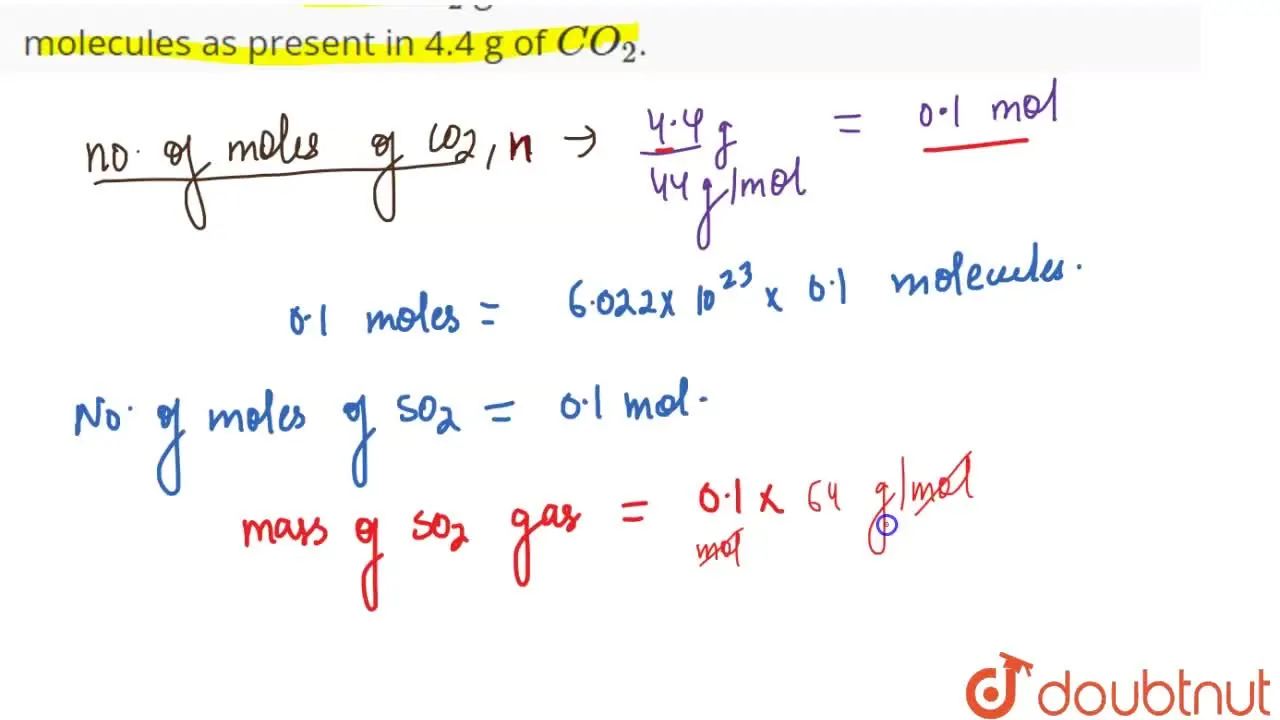
Calculate the mass of SO(2) gas which will contain the same number of

SOLVED: Solve the following numericals: (10M) 1. Calculate the number of particles in each of the following: a) 0.124 g of Mg atoms b) 18 g of O2 molecules 2. Convert into
How many number of moles are present in 24.088 x 10²³ numbers of sodium atoms? - Quora

Calculate mass of nitrogen n2 which contains same no Of molecules as are present in 4 4 g of - Science - Atoms and Molecules - 12397645

Best Answer] Calculate the number of molecules present in 4.4g of CO2. [At Mass: C=12, O=16 u, NA = 6.02

5 2.0 YUI marsn gas 42 Tv.v Bohosgene 23. The number of molecules present in 4.4g of CO, gas is [Jipmer-1990] 1) 6.023x1023 2) 5.023x102 3) 6.023x1024 4) 6.023x1022 of 1) +2 .
An Overview of Enabling Catalysts for Carbon Dioxide Conversion Aiming at the Two-carbon Target - Aerosol and Air Quality Research

15. A flaok contain 4.4g of coagas. Calculate: (a) How many moles of coa gás dots it contain (b) How many Molecules of Co2 gas are present in the sample © How
Which of the following contains the least number of molecules ? a) 4.4 g of CO2 b) 1.4 g of N2 c) 2 g of H2 d) 1 g of O2

The number of oxygen atoms in 4.4 g of CO2 is(a) 1.2 × 10^23 (b) 6 × 10^22 c) 6 × 10^23
2.4 Distribution of Molecular Speeds – University Physics Volume 2








