Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

By A Mystery Man Writer
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
The given graph represent the variation of z compressibility
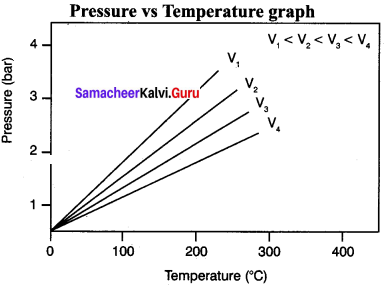
Samacheer Kalvi 11th Chemistry Solutions Chapter 6 Gaseous State – Samacheer Kalvi

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

01 Gaseous State#### PDF, PDF, Gases

Bansal classes chemistry study material for iit jee by S.Dharmaraj - Issuu

Compressibility factor Z - Gaseous State

Solved The plot below shows how compressibility factor (Z)
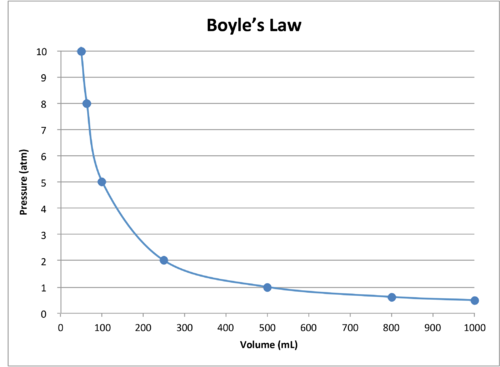
The Behavior of Gases Chemistry for Non-Majors

Revised+States+of+Matter
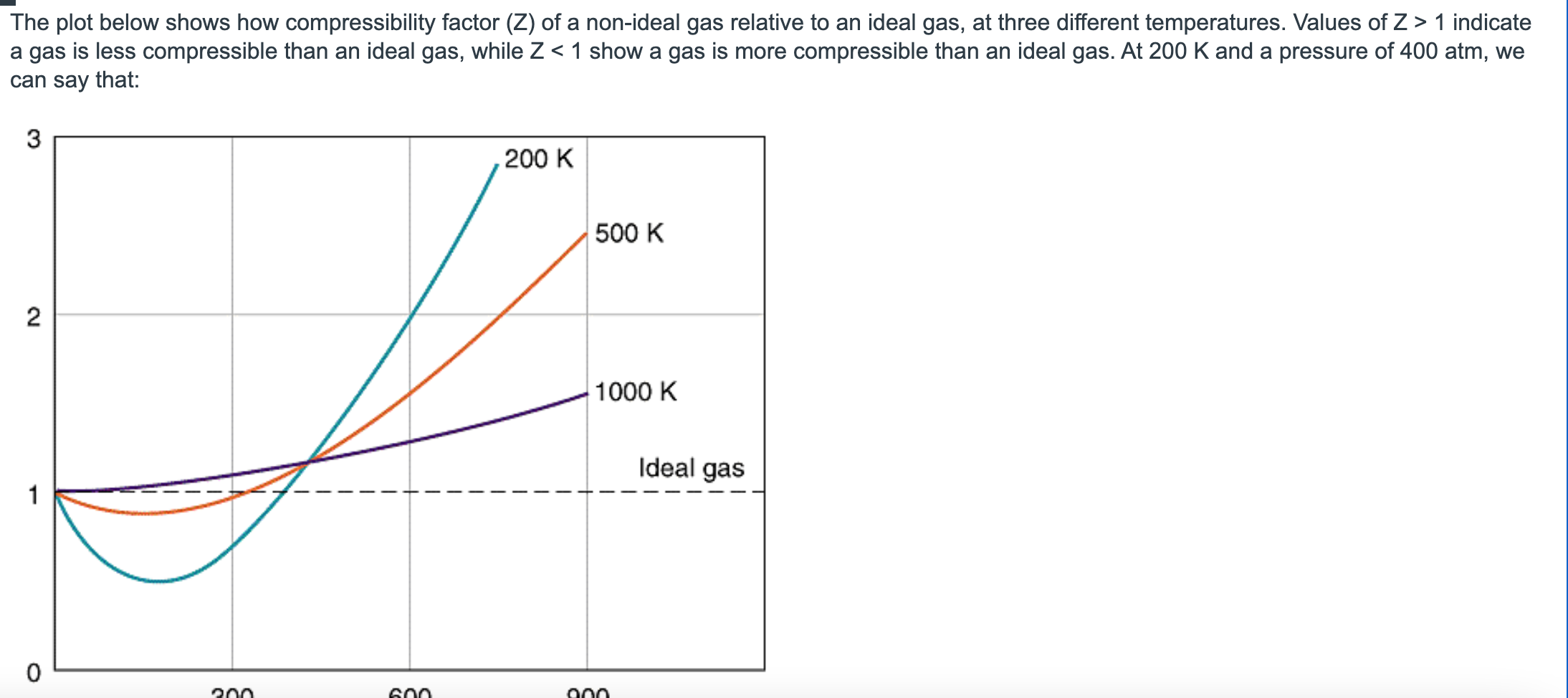
Solved The plot below shows how compressibility factor (Z)
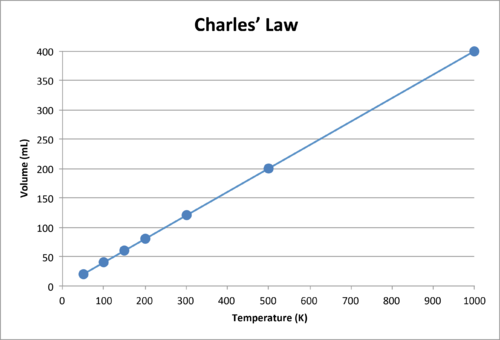
The Behavior of Gases Chemistry for Non-Majors
- Summary of Equations used to evaluate compressibility factor, z

- Gas Compressibility - an overview

- Solved QUESTION 3 Determine the compressibility

- Oil & Gas Softwares on X: Gas Compressibility Factor Calculator (Z-Factor) New App for #iPhone and #iPad #wellcontrol #drilling #Oil and #Gas #apps at / X

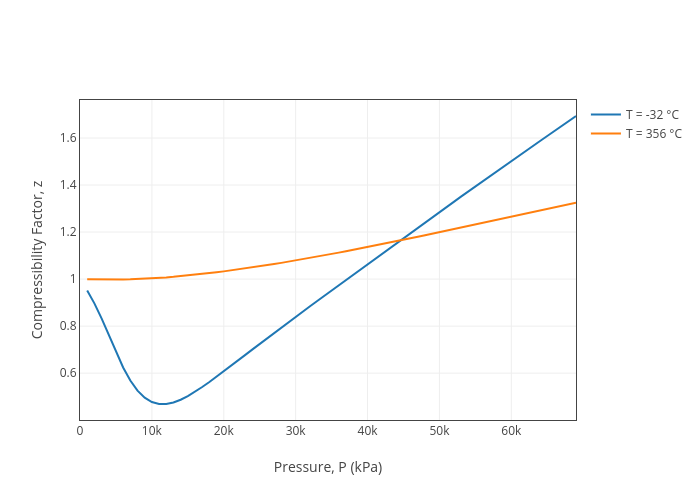
- Compressibility Factor, z vs Pressure, P (kPa), line chart made by Jdvani
- Girls Ruffle Skirt Leggings, Kids activewear, Yoga Dance Workout

- zanvin Sports bras for women ,plus size Yoga Bra Wireless Underwear,sleep bras for women Clearance Sale gifts for her,Light blue

- Lululemon Invisiwear Mid-rise Boyshort Underwear - Lavender Dew

- 3 Packs Toddler Little Boys Kids Underwear Breathable Cotton Dinosaur Boxer Briefs Size 4T 5T 6T 7T 8T

- Purple Satin Lingerie Set, Erotic Lingerie See Through, Boudoir Sheer Bra, Sexy Satin Panties - Canada
