20.If Z is a compressibility factor, van der Waals equation at low
By A Mystery Man Writer
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

If Z is a compressibility factor, van der Waals' equation at low

Deviations from ideal gas behaviour, intermolecular forces, Van

In the plot of Z (compressibility factor) vs P,Z attains a value of un

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
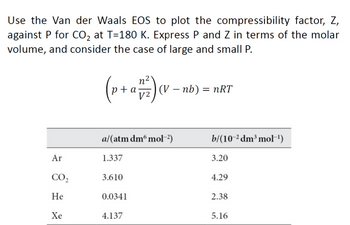
Answered: Use the Van der Waals EOS to plot the…

Solved APPENDIX Problem 1: Molar Volume and Compressibility
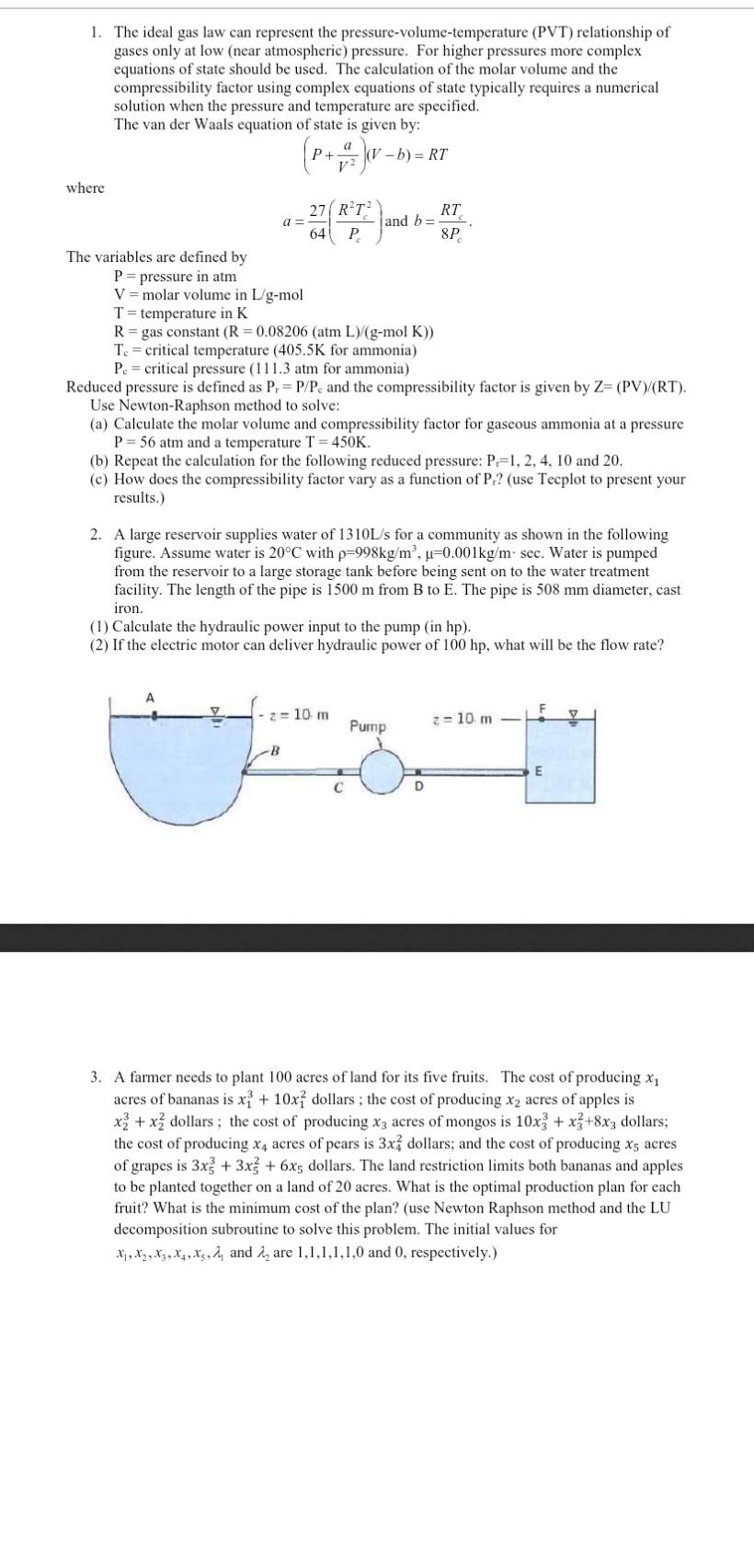
1. The ideal gas law can represent the
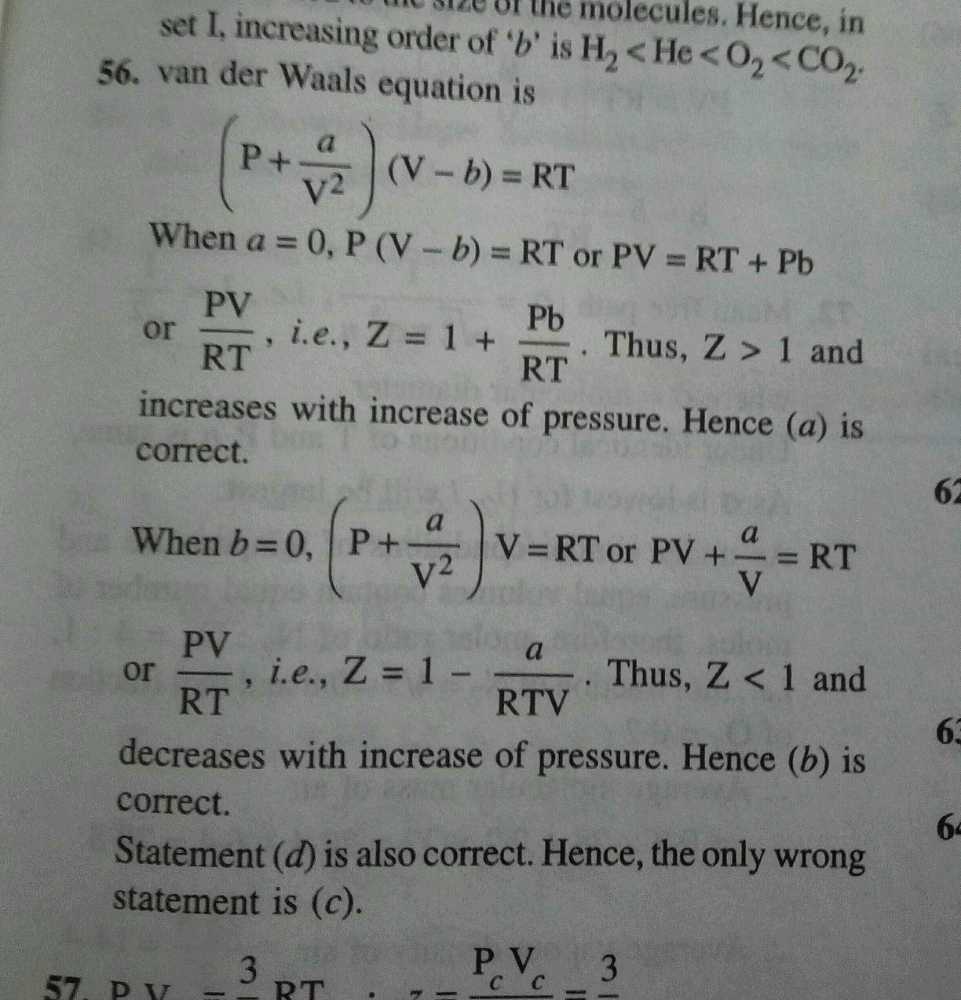
The given graph represents the variation of Z (compressibility factor

physical chemistry - Why do some gases have lower value of Z for a

physical chemistry - Why do some gases have lower value of Z for a

Equation of state (excess compressibility factor Z À1 ¼ PV/(NkT) À

Compressibility factor - Wikipedia

Solved 1. (10 points) Calculate the compressibility factor

If Z is a compressibility factor, van der Waals equation at low

Real gases 1.4 Molecular interactions 1.5 The van de Waals
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- Compressibility Factor Calculator - File Exchange - MATLAB Central

- Solved We showed, for a van der Waals gas, that the
- Compressibility Factor Charts - Wolfram Demonstrations Project

- Physical Chemistry The Compression Factor (Z) [w/1 example

- Malaysian brides return to elegant, romantic hairstyles for their weddings

- Tween Matte Flex Super High Waist Long Legging - Black - ShopperBoard

- Kimono Jiu Jitsu Koral Novo MKM Harmonik Branco

- Montelle Modal Bust Support Nightgown

- Vanity Fair Lightly Padded Underwire Bra 44C

- Wholesale Order Form & ReOrder - Customers can bulk order products from a quick Order Form!

- 3D Posy Double-Layered Mesh Midi Skirt in Caramel - Retro, Indie

- Scratch Wire Brush By Kemper Tools

- Buds Fitness - Quick drying, comfort fit t shirt

- Corset Slimming Gothic Style Underbust Bustier Tops Body Shaper 20

