Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

By A Mystery Man Writer

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

Real Gas Behavior The Compression Factor (Z) [Example #2]

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

ANSWERED] Q 32 Compressibility factor Z of a gas is given as Z pV nRT - Kunduz

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

Deviation from ideal gas behaviour

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics


Solved] The compressibility factor for an ideal gas is
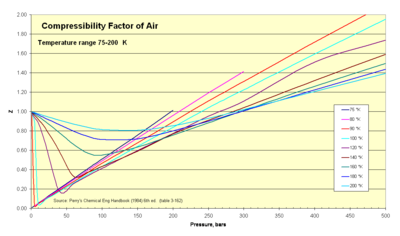
Gas - Wikipedia
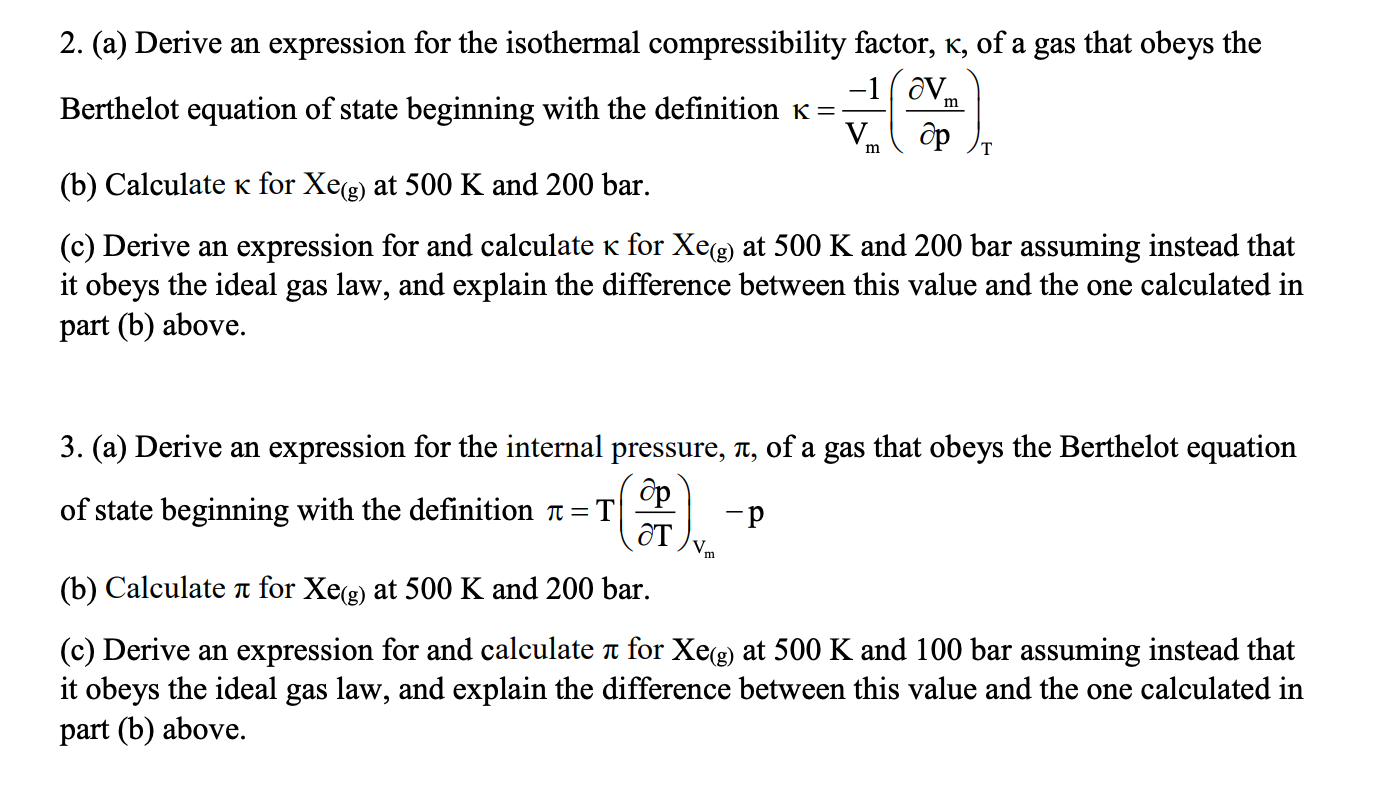
- Solved 2. (a) Derive an expression for the isothermal
- Which of the following statements is/are correct? (a) all real gases are less compressible

- Compressibility Factor Z Important Concepts and Tips for JEE Main

- the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians

- Real gasses For an ideal gas, the compressibility factor Z = PV