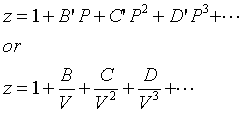At a high pressure, the compressibility factor (Z) of a real gas is us

By A Mystery Man Writer
At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.

At a high pressure, the compressibility factor (Z) of a real gas is us

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange

Compressibility Chart - an overview

COMPRESSIBILITY FACTOR
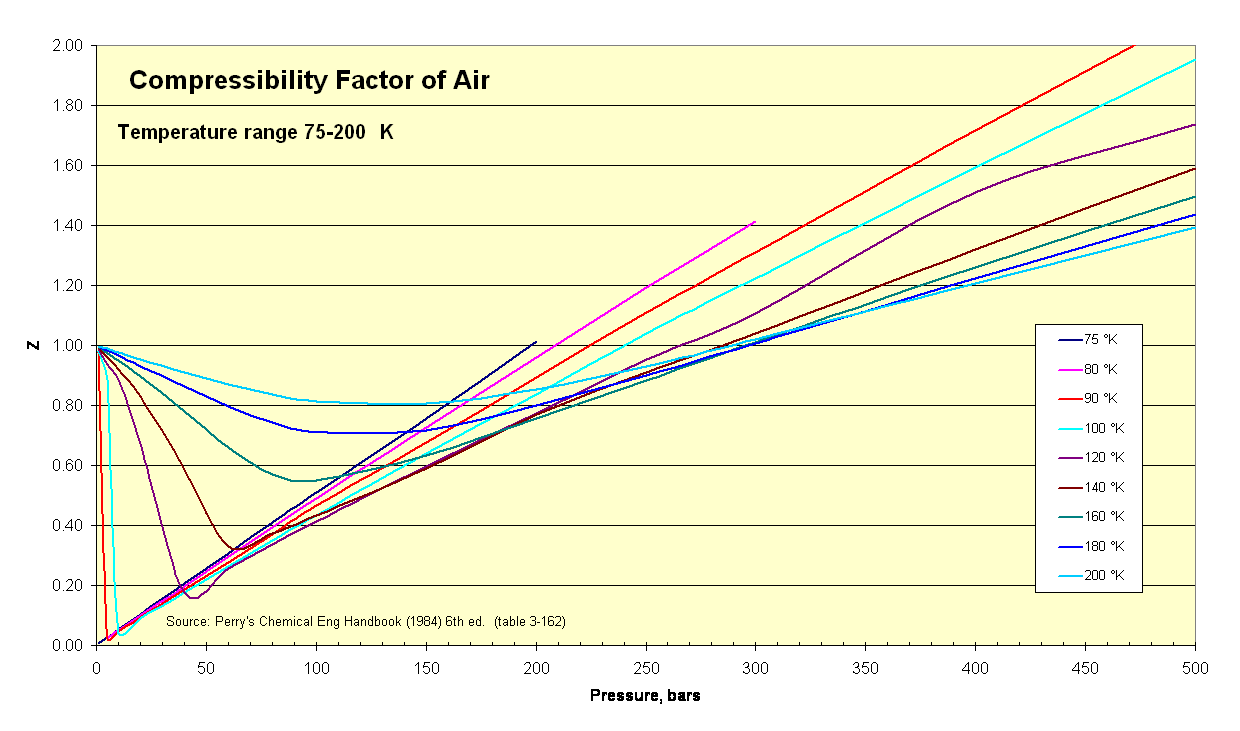
Air Compressibility Factor Table - EnggCyclopedia
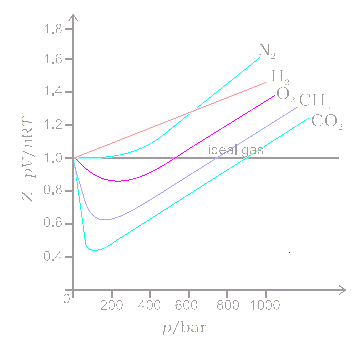
Van der waals equation: Derivation, Explanation

The role of the compressibility factor Z in describing the volumetric behavior of gases
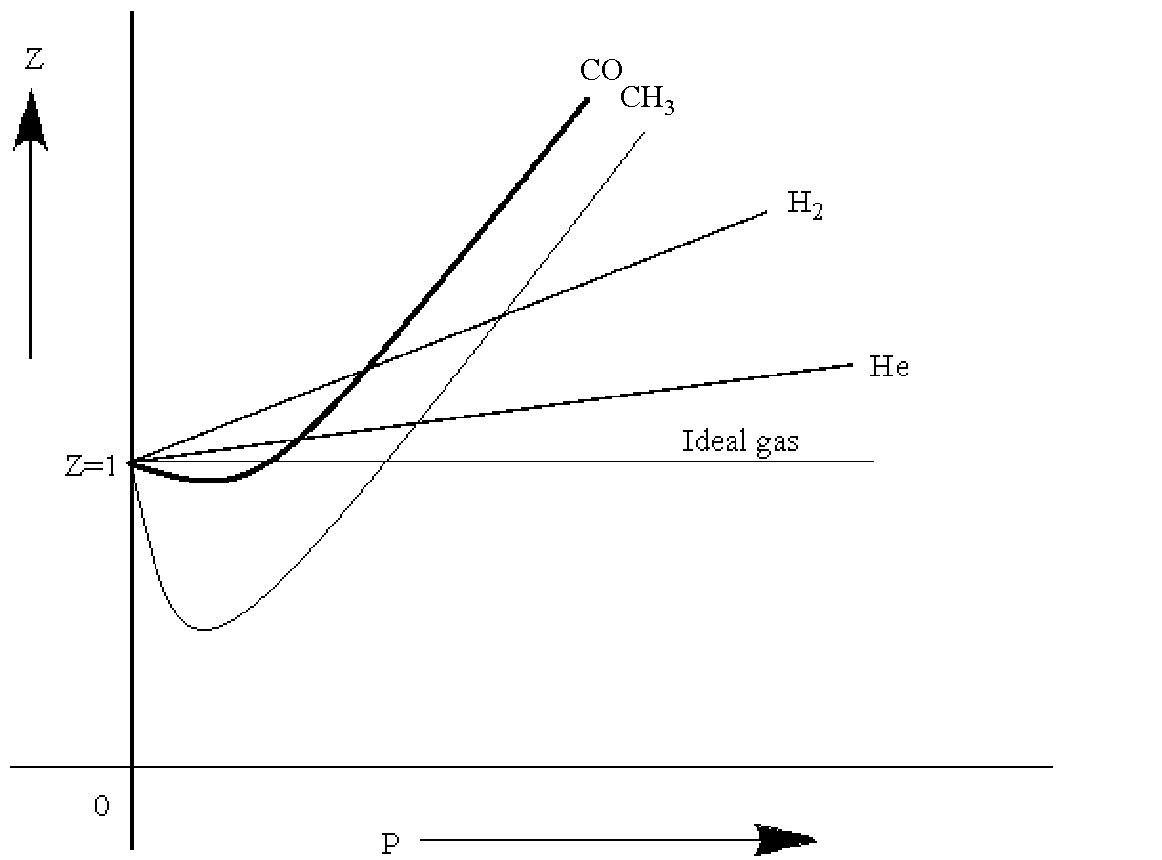
Boyle's temperature or Boyle point is the temperature at which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between the compressibility

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

Compressibility factor Z - Gaseous State

3.2 g of oxygen gas is placed in a vessel of 10 litre at 1000 K so tha

Real gas z-Factor chart [2] Download Scientific Diagram

Ch2, Lesson E, Page 9 - Generalized Compressibility Chart
- Power UltraSculpt High Waist 7/8 Workout Leggings Colour Block - Blue Pixel Leopard Print, Women's Leggings
- Generic 1PCS Leg Support Socks Calf Compression Sleeves Leg
/product/03/1462362/2.jpg?2281)
- Wmbra Posture Correcting Bra, Wireless Push-Up Ergonomic Comfort

- Quick brake 114-5023 repair kit caliper brake
- Faja Medica Post Operatoria Cirujia Liposuccion Thong Body Post-surgey Beige