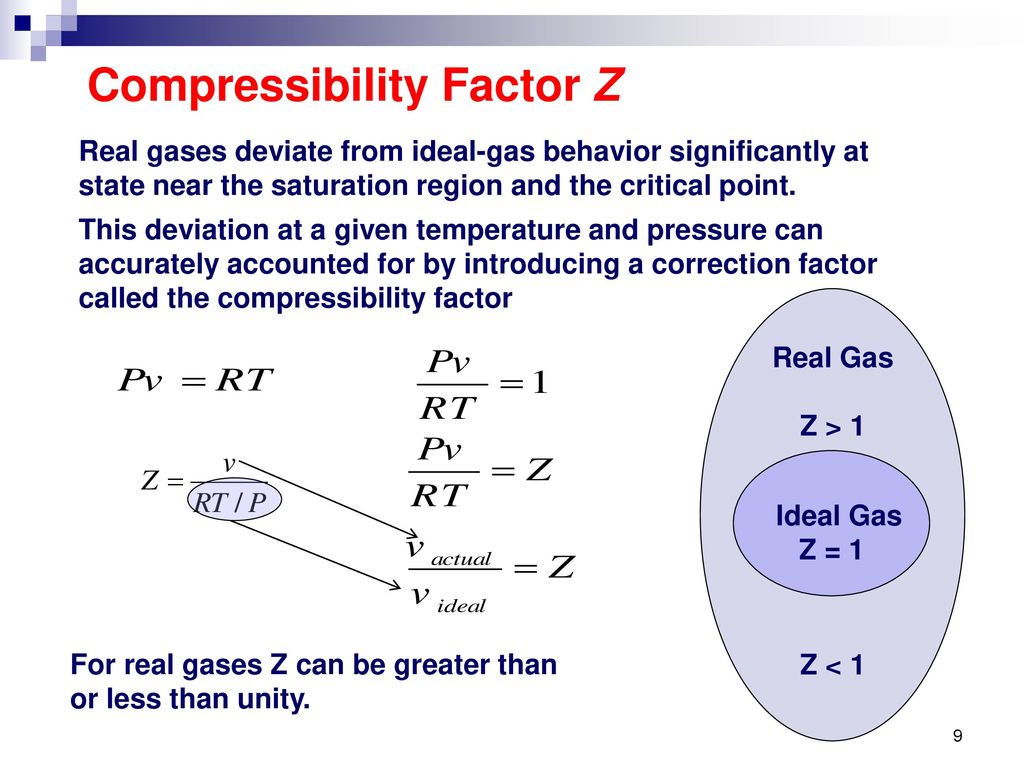
The compressibility factor Z for an ideal gas will be

By A Mystery Man Writer
The compressibility factor Z for an ideal gas will be
Ideal gases and real gases are compressible or not compressible what is the compressible factor for real gases and ideal gases.

Plot of experimental measurements of the z-factor
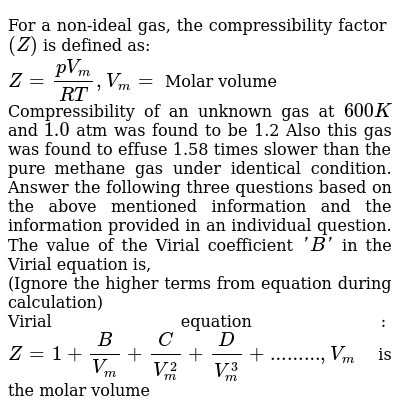
For a non-ideal gas, the compressibility factor (Z) is defined as: Z

Thermodynamic Properties Property Table w Property Table -- from direct measurement w Equation of State w Equation of State -- any equations that relates. - ppt download

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
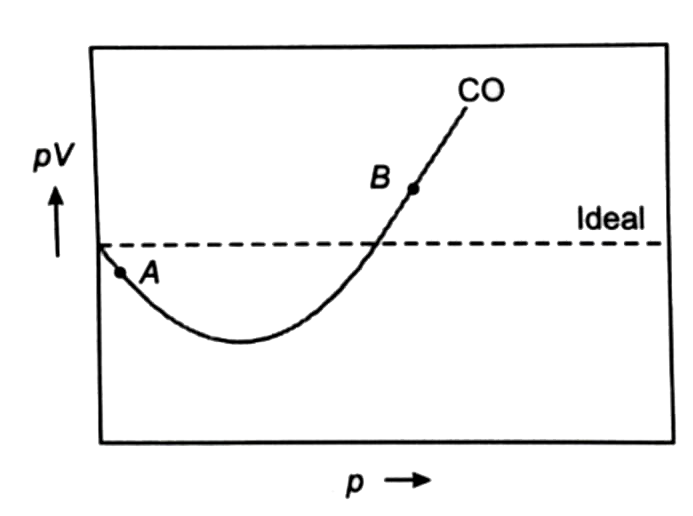
For Co, isotherm is of the type as shown. Near point A, compressibilit

Statement-1 is correct, Statement-2 is incorrect.

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
The Ideal Gas. - ppt download

Chemistry Desk: Effect of Pressure
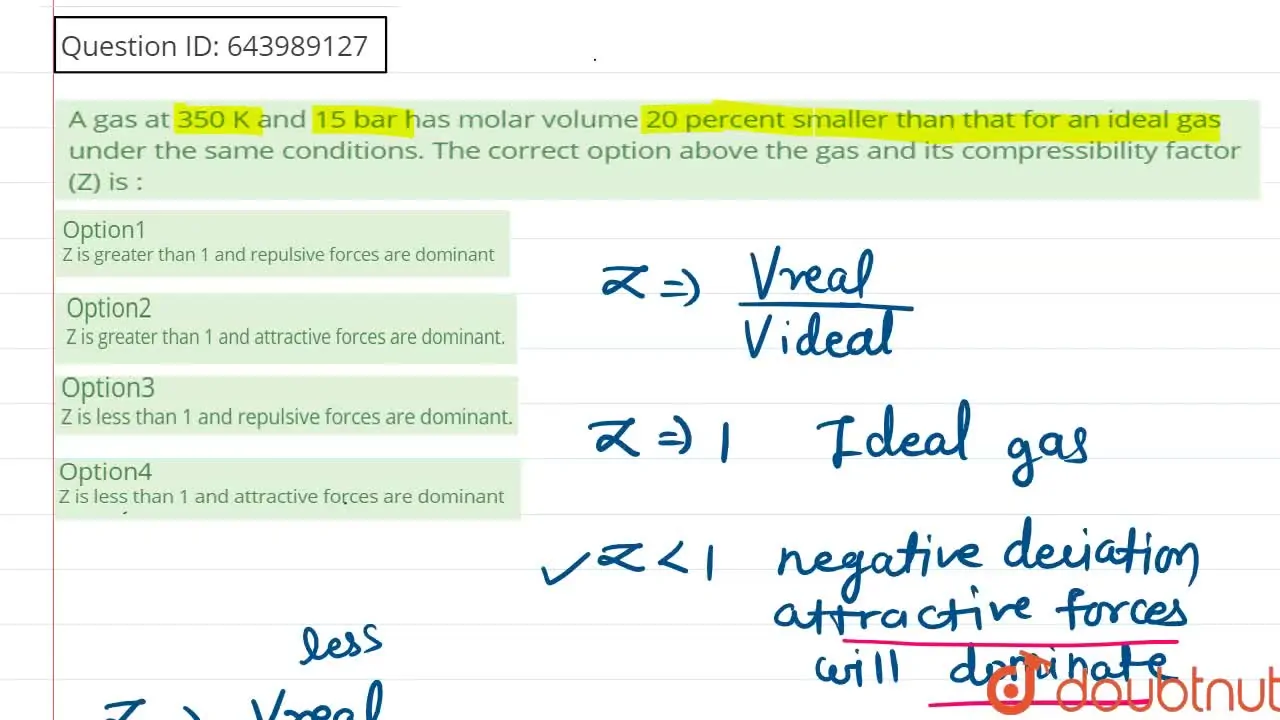
Z is less than 1 and repulsive forces are dominant.

Compressibility factor, Z of a gas is given as `Z=(pV)/(nRT)` (i) What is the value of Z for an

Compressibility Factor Z Important Concepts and Tips for JEE Main
- Summary of Equations used to evaluate compressibility factor, z

- Solved Show that the compressibility factor of van der Waals

- Cubic Equation of State for the Compressibility Factor - Wolfram Demonstrations Project
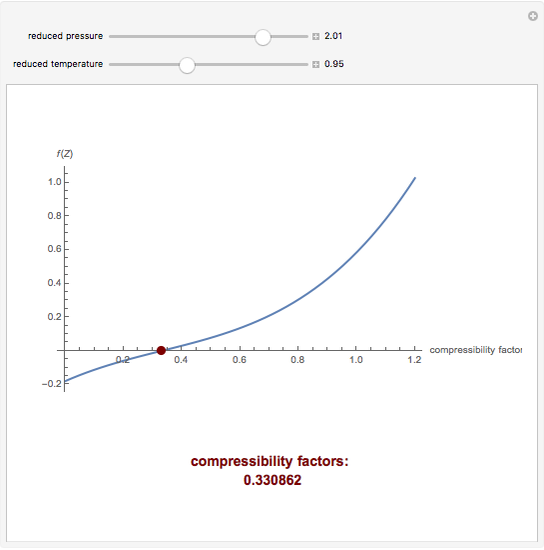
- Compressibility factors of air using improved virial equation and

- The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
- Garments Shop Flex Design, Kapade Ki Dukan Ka Banner, Cloth Collection Shop, Fashion Shop Banner

- Jockey Set of 4 Soft Touch Breathe Briefs
- Khaki Field Titanium Auto

- Cloud Seamless Cut Out Bra - Fabletics

- Big Clearance! Juebong Sexy Side Slit Leggings for Women Plus Size Wide Leg Pants Women Casual Summer Long Loose Yoga Pants

