The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement

By A Mystery Man Writer

thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange

6.3: Van der Waals and Other Gases - Physics LibreTexts

The given graph represents the variation of Z(compressibility factor =- PV nRT ) versus P, three real gases A, B and C. Identify the only incorrect statement. Ideal gas P (atm) (A)

The given graph represent the variations of Z (compressibility factor (Z)=dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may

Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect

Why does ammonia deviate from ideal gas? - Quora

Van der Waals equation - Wikipedia
What is the significance of the curve part in Z vs. P graph of compressibility of a gas? - Quora
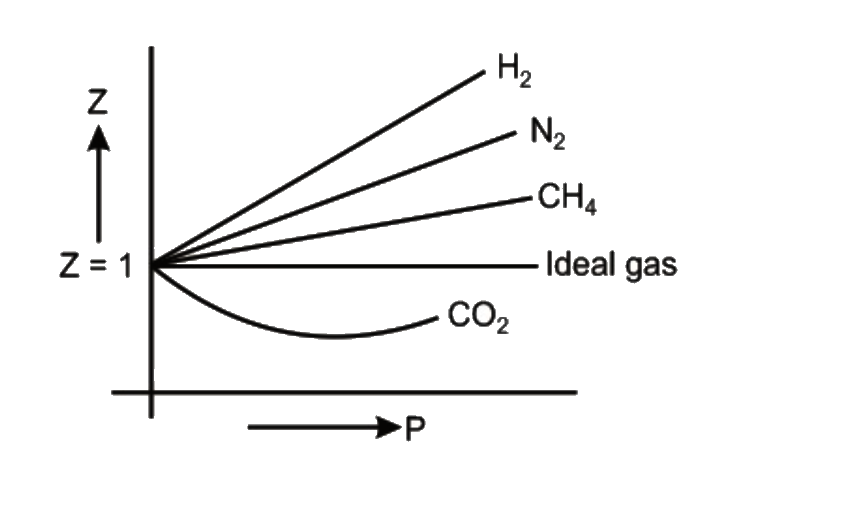
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What
Why does ammonia deviate from ideal gas? - Quora

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
- Compressibility Factor Calculator - File Exchange - MATLAB Central

- Determine Compressibility of Gases

- The role of the compressibility factor Z in describing the
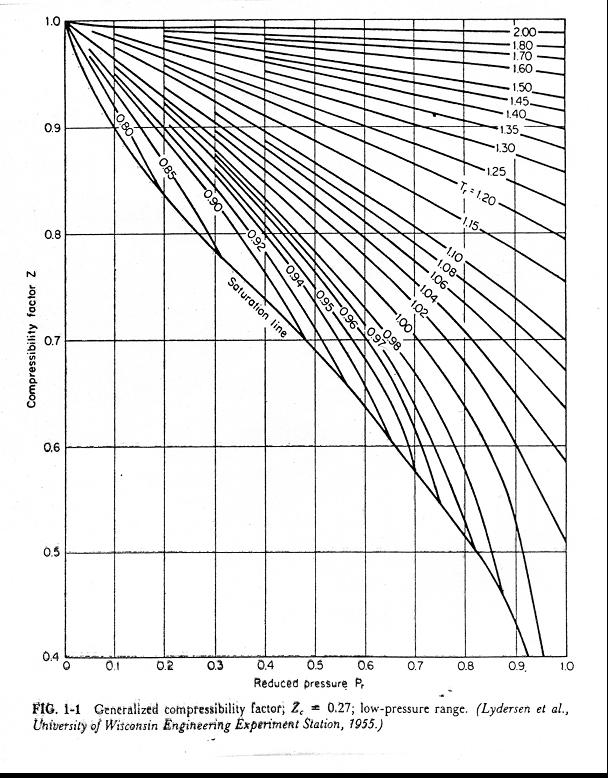
- New explicit correlation for the compressibility factor of natural
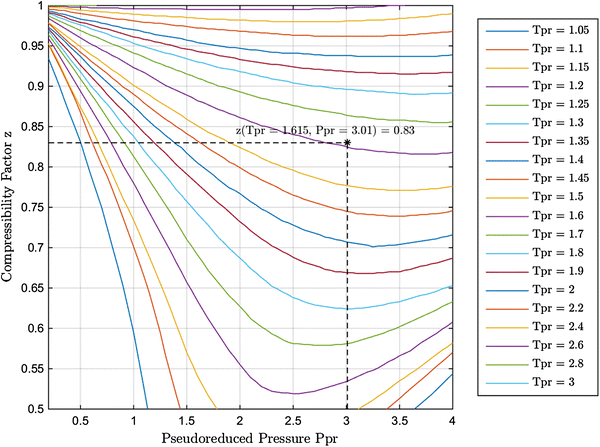
- plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange




