If `Z` is a compressibility factor, van der Waals' equation at low

By A Mystery Man Writer
If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

plotting - How to plot Compressibility factor Z vs Pressure P
Thermo] Derivation of compressibility factor vs reduced pressure

image.slidesharecdn.com/unit10realgasesvdwfl14fina

66. If z is the compressibility factor, van der Waals equation low

Van der Waals equation, when pressure correction is ignored, one

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Behaviour of Real Gases, PDF, Gases

If `Z` is a compressibility factor, van der Waals' equation at low
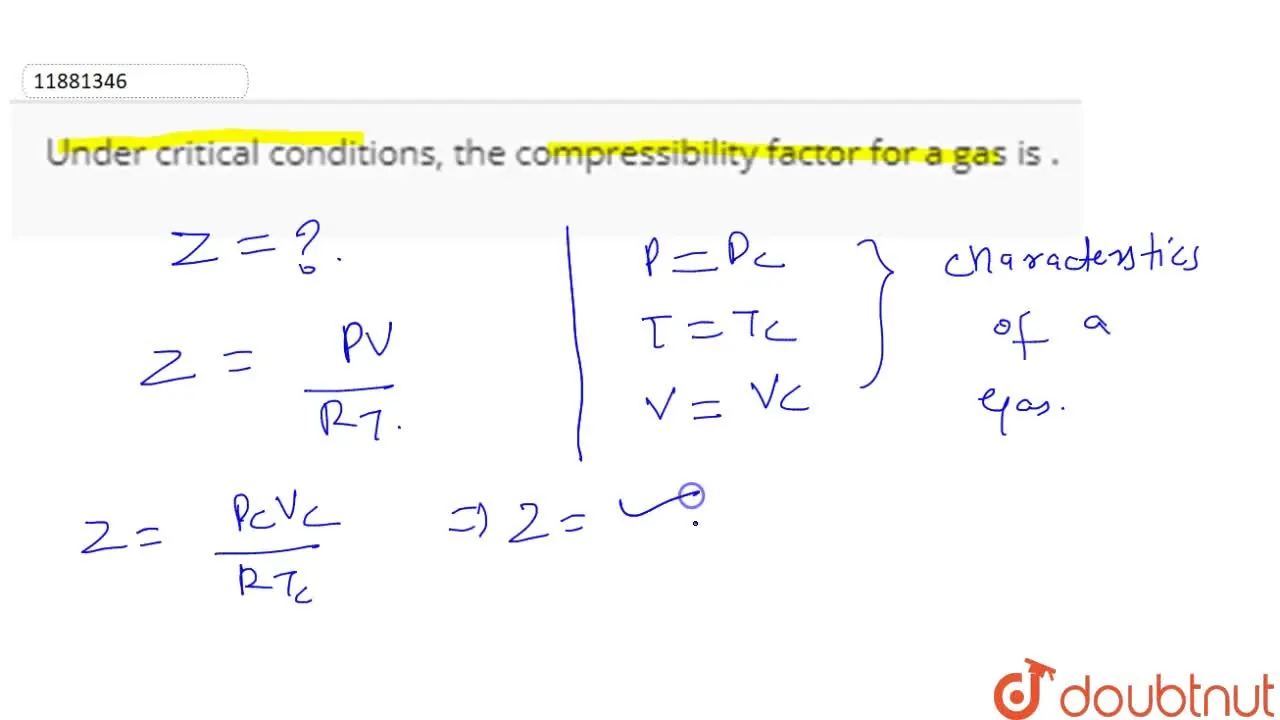
Under critical conditions, the compressibility factor for a gas is .
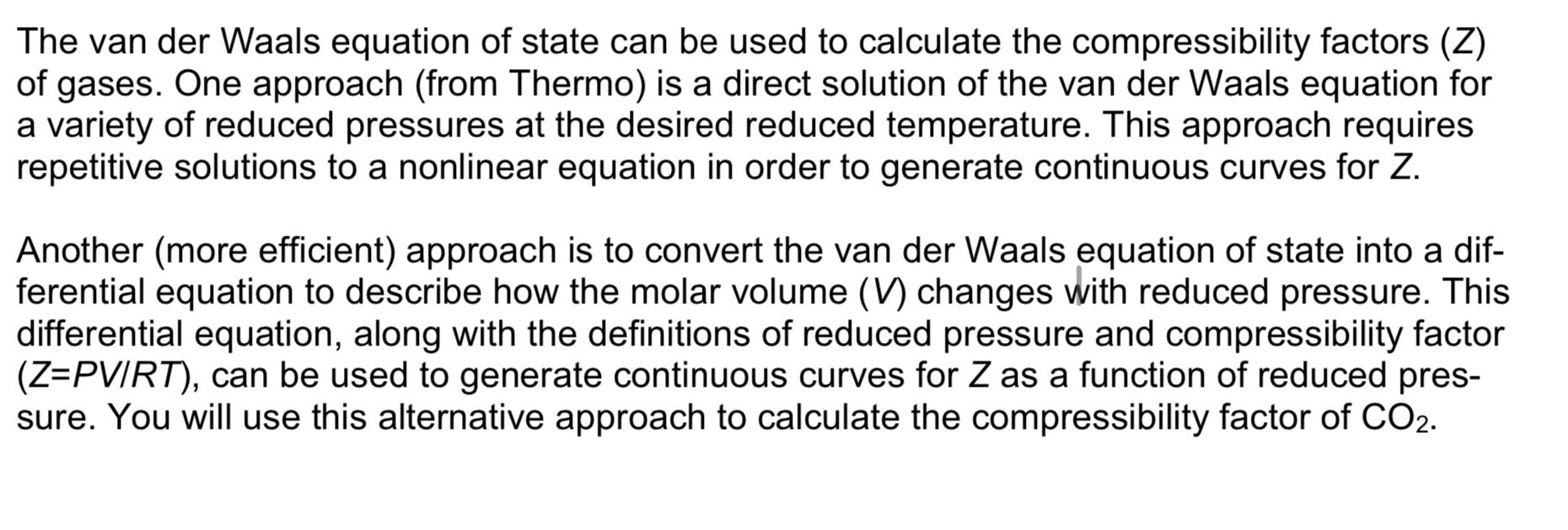
Solved The van der Waals equation of state can be used to

1.7: Connecting the van der Waals and the viral equations: the

Derivation of Van Der Waals Equation

In the plot of Z (compressibility factor) vs P,Z attains a value of un
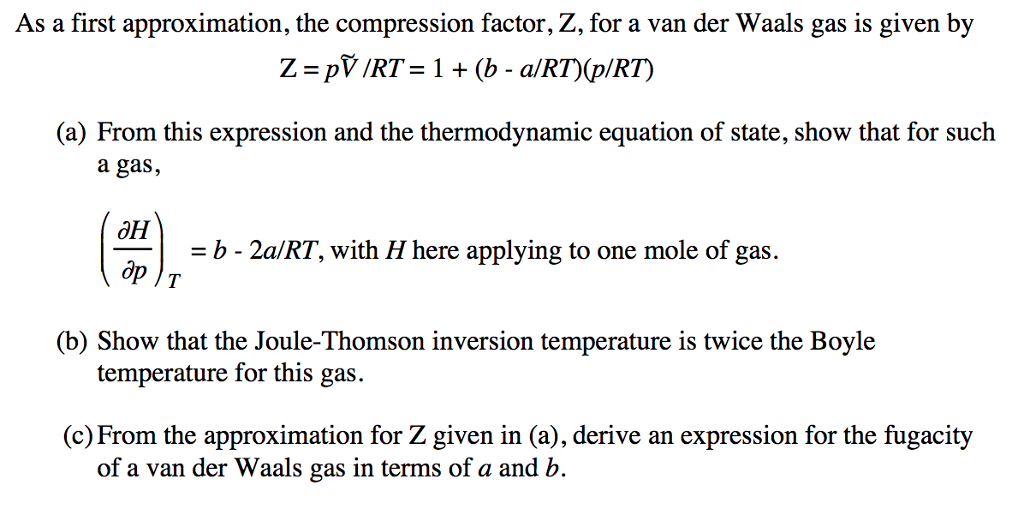
- Solved As a first approximation, the compression factor, Z
- Solved 1. The compression factor, Z of a gas is 0.625. Which
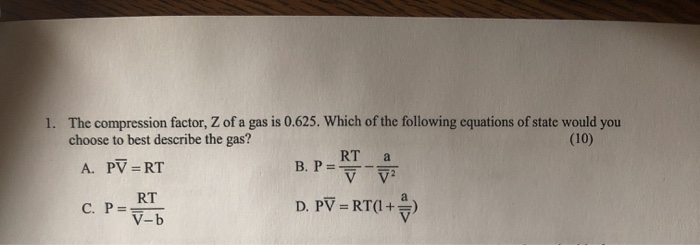
- Compression Factor and Fugacity

- Real gases

- At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

- BootYo! PackYo! Utility Straps/Cinch lash Strap with

- Viscose Spandex Stretch Single Jersey Fabric, per Metre 170 Cms

- Sacramento, CA Travel Guide- Top Hotels, Restaurants, Vacations, Sightseeing in Sacramento- Hotel Search by Hotel & Travel Index: Travel Weekly

- Calvin Klein Underwear Modern Cotton Padded Triangle In Black. - Size L (Also In M, S) for Women
:quality(80):fill(white)/https:%2F%2Fis4.revolveassets.com%2Fimages%2Fp4%2Fn%2Fd%2FCKUD-WI177_V1.jpg)
- Boody Bamboo Underwear - Full Brief
