Structure of YF1. a Structure of YF1 in its dark-adapted state as

By A Mystery Man Writer
Download scientific diagram | Structure of YF1. a Structure of YF1 in its dark-adapted state as resolved by X-ray crystallography 13. The location of the different domains, of the flavin mononucleotide (FMN), of the cofactor adenosine diphosphate (ADP), and of the phosphoaccepting histidine 161 are indicated. b Light induced conformational changes of the LOV photosensor domain refined from X-ray solution scattering 22. The changes are maximal at the C-termini that feed into the Jα helices (dashed arrows). The coloring is according to the root mean square deviation of the alpha carbons from publication: Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase | Sensor histidine kinases are central to sensing in bacteria and in plants. They usually contain sensor, linker, and kinase modules and the structure of many of these components is known. However, it is unclear how the kinase module is structurally regulated. Here, we use | Secondary Protein Structure, Bacterial Proteins and Protein Conformation | ResearchGate, the professional network for scientists.

IJMS, Free Full-Text

Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase

Signaling States of a Short Blue-Light Photoreceptor Protein PpSB1

Structure of the YtvA STAS (a) and LOV (b) domains with the position of
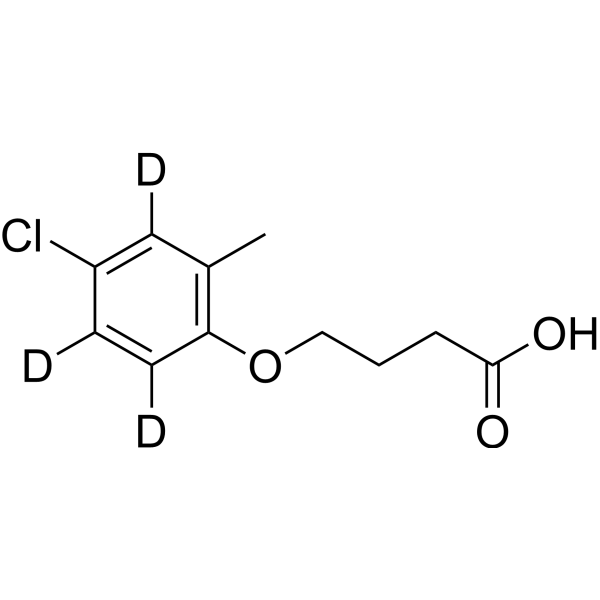
4,4'-Dihydroxydiphenylmethane-d10

Structures of theaflavins. a TF1, b TF2B, c TF2A, d TF3
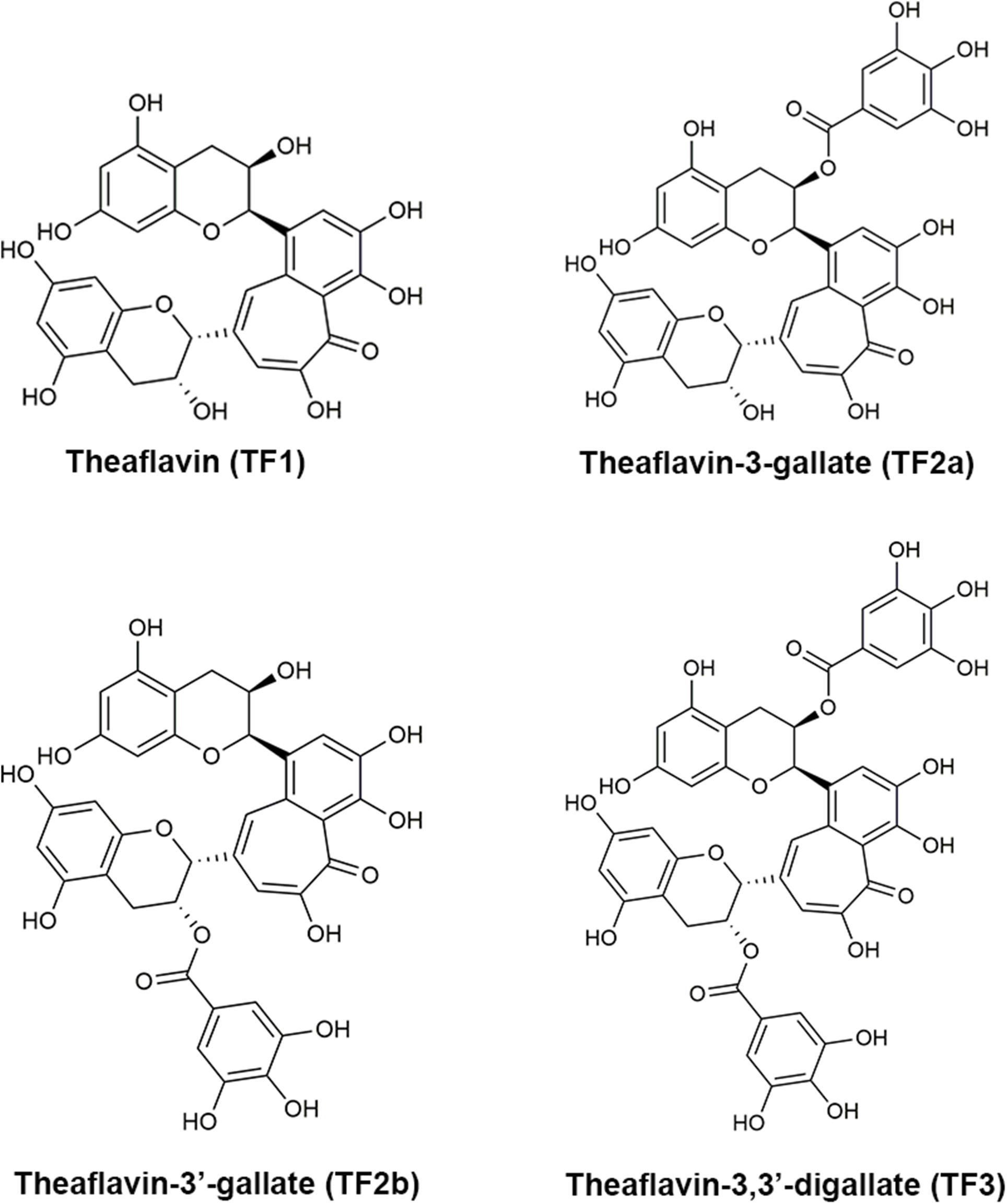
Frontiers Theaflavin-3,3′-Digallate Suppresses Biofilm Formation, Acid Production, and Acid Tolerance in Streptococcus mutans by Targeting Virulence Factors

Ralph DIENSTHUBER, Dr., Humboldt-Universität zu Berlin, Berlin, HU Berlin, Department of Biology

PDF) Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase

Structural photoactivation of YF1. Blue light causes sequential

Crystal structure of LOV-PAS and LOV-PAS-HK. (A) Ribbon diagram of
- State and Trends in Adaptation Report 2022 - Global Center on Adaptation

- Adapted State High-Rise Jogger *Full Length, Women's Joggers
- Lululemon athletica Adapted State High-Rise Jogger *Full Length, Women's Joggers

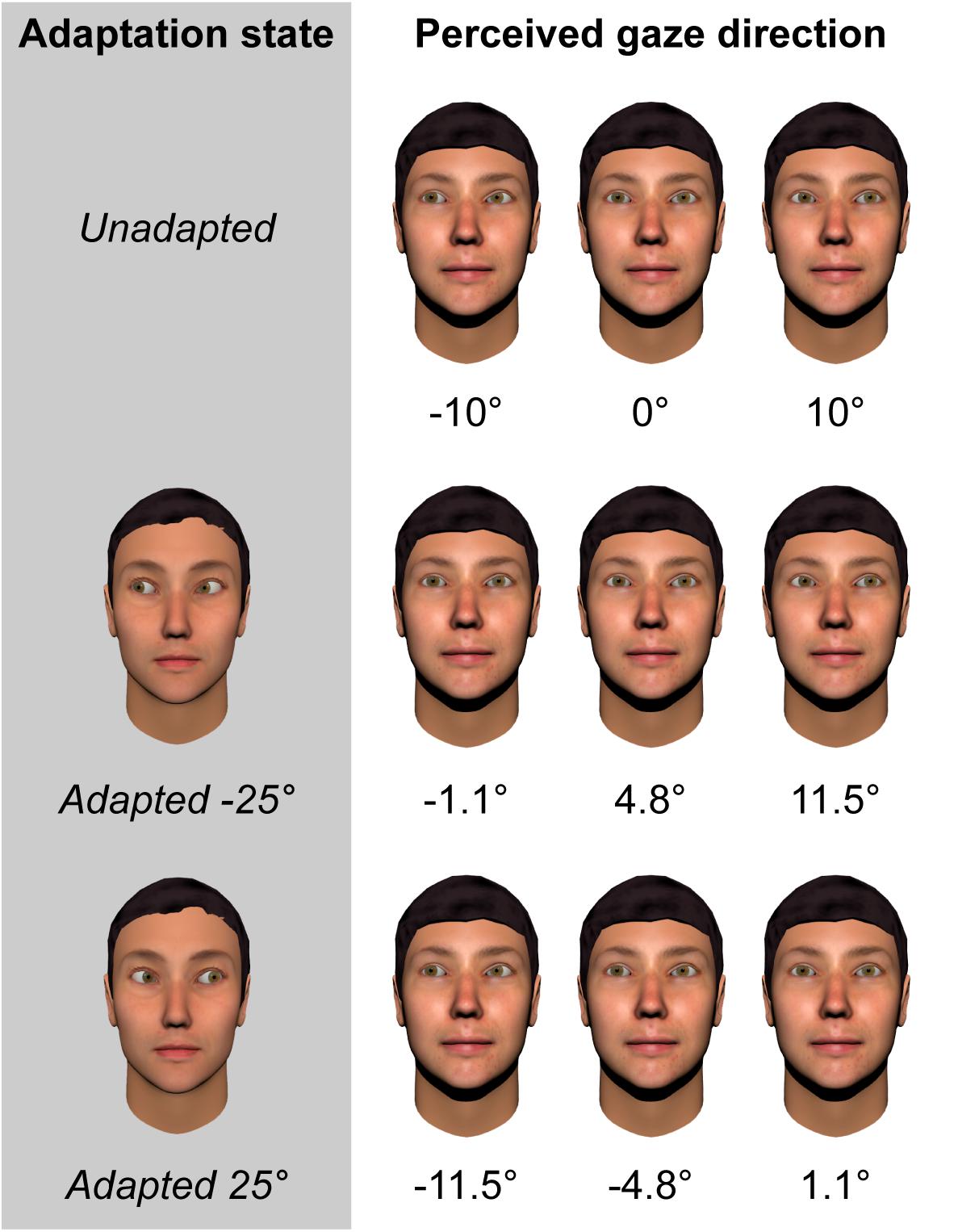
- Frontiers Adaptation to the Direction of Others' Gaze: A Review
- Lululemon Adapted State High-Rise Jogger *Full Length - Dramatic Magenta - lulu fanatics

- Bamboo bras ❤️

- Peloton Sunrise Fade High Rise Athletic Compression Leggings Women's Medium
- 390+ Supermom Stock Illustrations, Royalty-Free Vector Graphics & Clip Art - iStock

- Chlorine Resistant Tummy Control Tankini Top Tropical Line

- The North Face Jaqueta preta Evolve II Triclimate® - Esdemarca Loja moda, calçados e acessórios - melhores marcas de calçados e calçados de grife

