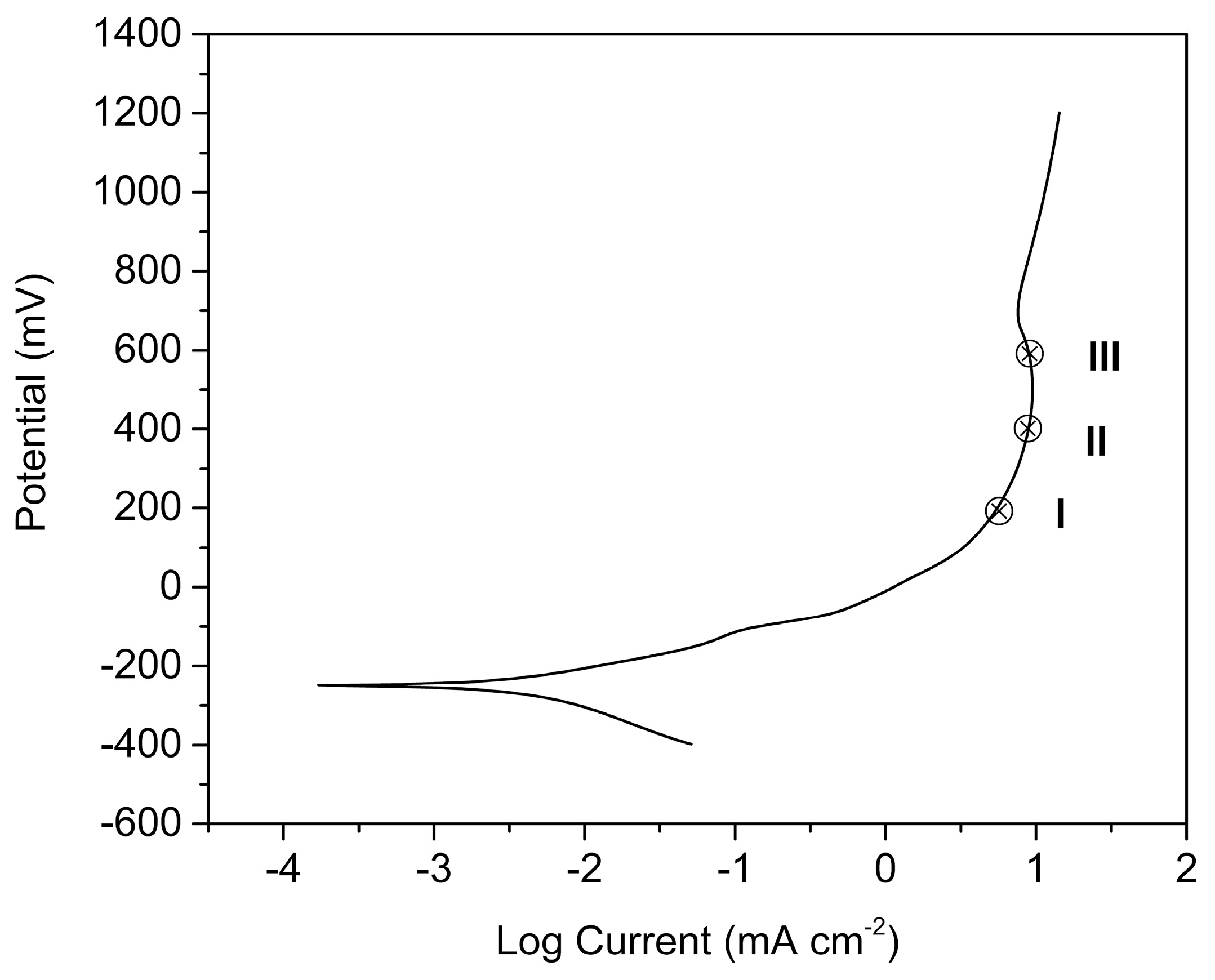
Typical steady-state polarization curve obtained for a gold electrode

By A Mystery Man Writer
Download scientific diagram | Typical steady-state polarization curve obtained for a gold electrode (length 0.50 cm, width 0.60 cm) in a channel-type cell (halfheight 0.16 cm, width, 1.2 cm) in a 10 mM Na2SO3 in 0.50 M phosphate buffer solution (pH ) 5.25) at a flow rate of 0.32 mL/s, i.e., vo ) 2.5 cm/s (solid circles, right ordinate). The open squares (left ordinate) are the absorbances at 316 nm measured downstream of the electrode as a function of the applied potential. The ordinates were scaled to show the direct proportionality between the current and the amount of dithionite detected spectroscopically in the range-0.50 to-0.75 V (see text for details). The lines represent a polynomial fit to the data. from publication: In Situ Spectroscopic Determination of Faradaic Efficiencies in Systems with Forced Convection under Steady State: Electroreduction of Bisulfite to Dithionite on Gold in an Aqueous Electrolyte | The reduction of bisulfite on Au electrodes in buffered aqueous solutions (pH = 5.25) was examined by in situ near-normal-incidence UV−visible reflection absorption spectroscopy on a rotating disk electrode (RDE) and in situ transmission UV−visible spectroscopy downstream | In Situ, Spectroelectrochemistry and Efficiency | ResearchGate, the professional network for scientists.

Typical steady-state polarization curve obtained for a gold electrode

Electrode polarization curves. (a) cathode i-V curves showing the

Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers

A) Representative polarization curve (red) and power curve (blue)

Typical steady-state polarization curve obtained for a gold electrode

A) Polarization curves (iR corrected, capacitance corrected) of the

Circularly polarized light-sensitive, hot electron transistor with chiral plasmonic nanoparticles

PDF) In Situ Spectroscopic Determination of Faradaic Efficiencies

PDF) In Situ Spectroscopic Determination of Faradaic Efficiencies

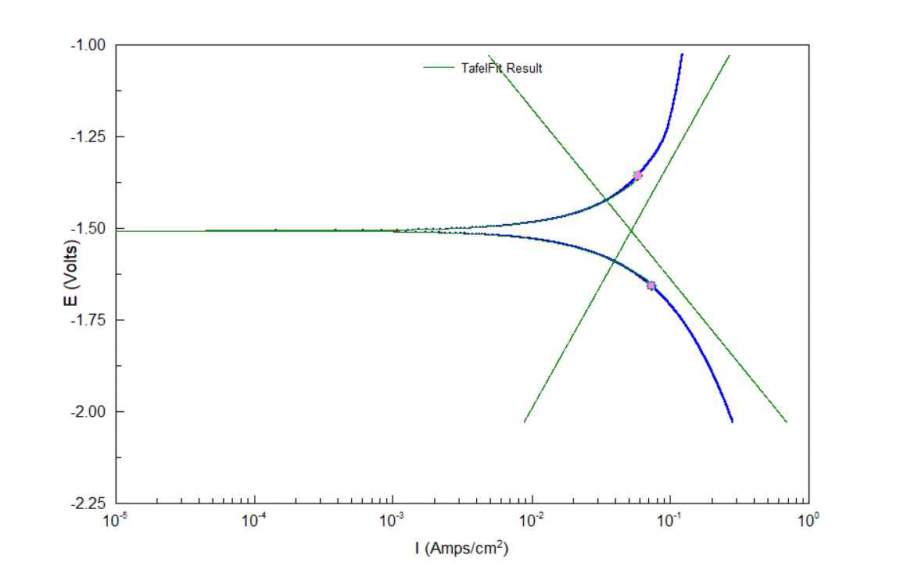
Electrochem Eng L03-08 Polarization curve and example for an electrode reaction