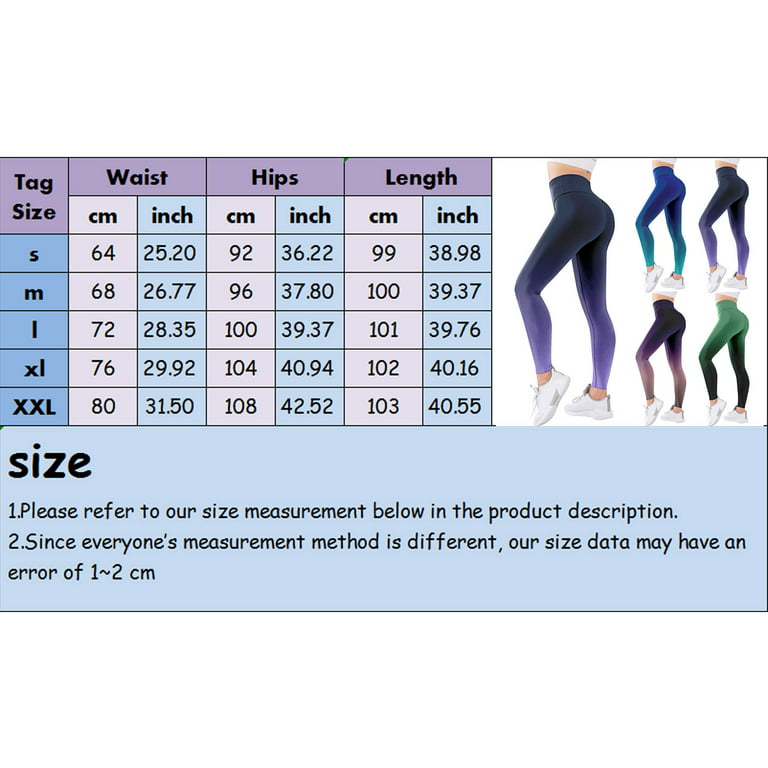
Saturday, Oct 05 2024
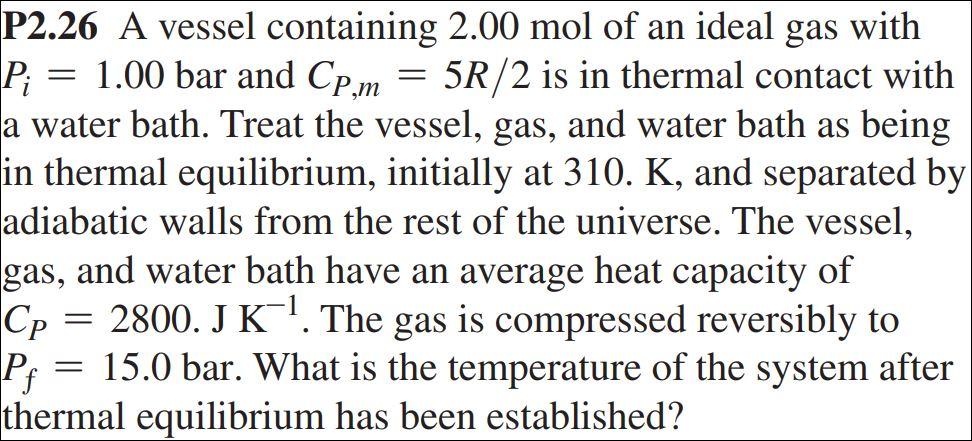
Solved P2.26 A vessel containing 2.00 mol of an ideal gas
By A Mystery Man Writer

A gaseous mixture enclosed in a vessel of volume V consists of one mole of a gas A with γ=5 / 3 a

If an ideal gas is compressed during isothermal process then :- P (
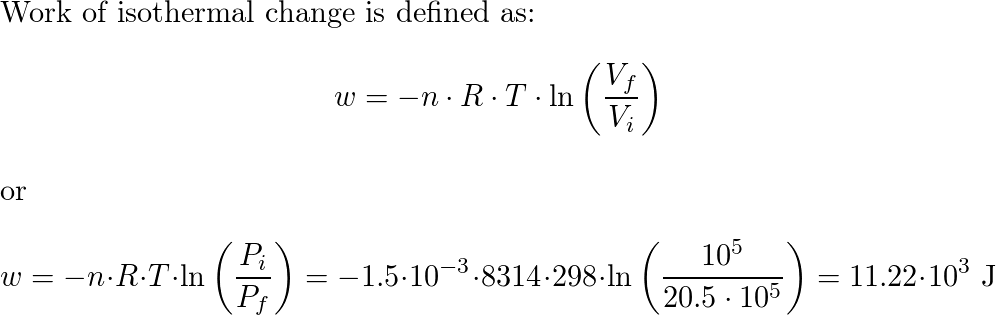

A vessel contains 2 gms. of gas and the pressure is 40 cm of Hg. under the same conditions. 1 gm. of gas is introduced into the vessel. the pressure will be

Combined Gas Law - Chemistry

Answered: What is the volume of 2.00 moles of…

Campbell Tip of the Month

Chapter_132-27-2011-90257PM

Answered: A flexible container at an initial…
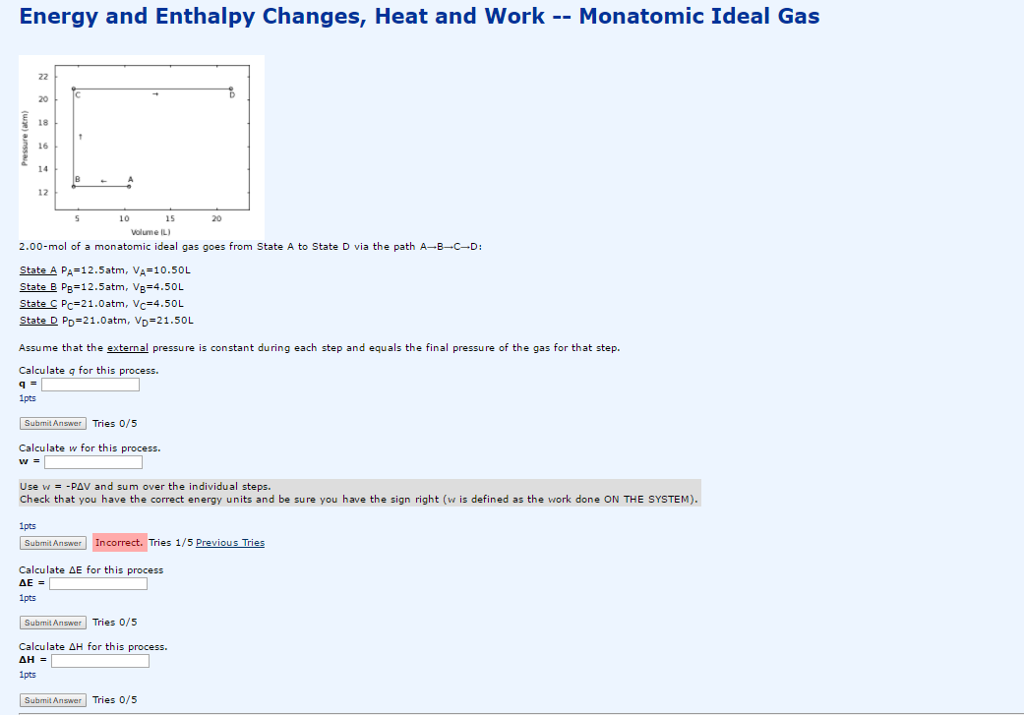
Solved 2.00-mol of a monatomic ideal gas goes from State A
Related searches
- The Ideal Timing for Pi Network Mainnet Launch: Selecting Dates that Ignite Enthusiasm - hokanews

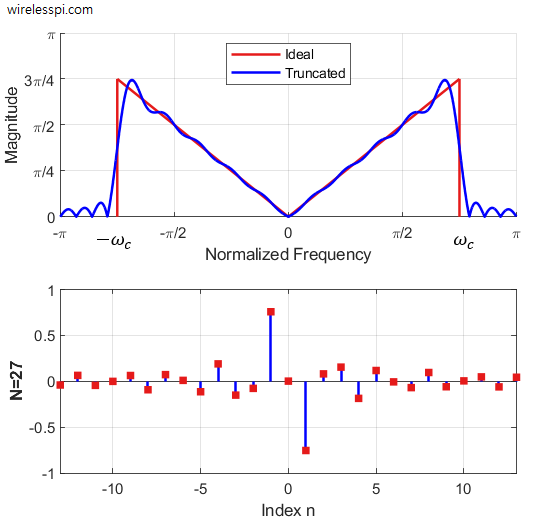
- Design of a Discrete-Time Differentiator
- IDEAL Digital Insulation Meter with PI, DAR, Remote Probe 61-797 - The Home Depot

- Lipoelastic PI Perfect Post Surgery Bra - Natural

- Igreja Parque Ideal – PI – PORTAL 300 DE GIDEÃO

©2016-2024, reintegratieinactie.nl, Inc. or its affiliates