Solved A diprotic base (B) has pKb values of 3.08 (pKb1) and
By A Mystery Man Writer

The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points

CHEM 14.1-14.6 except 14.5 Flashcards

Calculating the pH of a weak diprotic acid solution

OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo
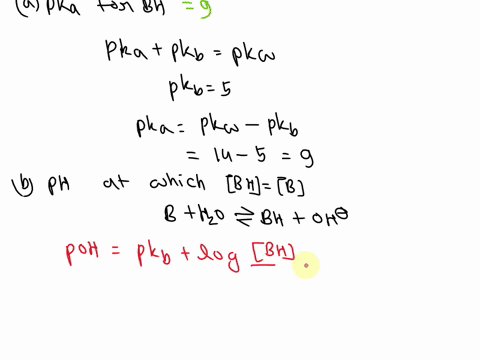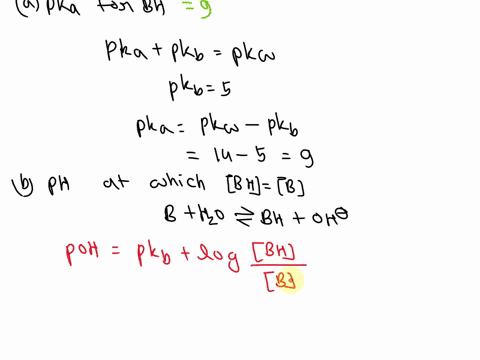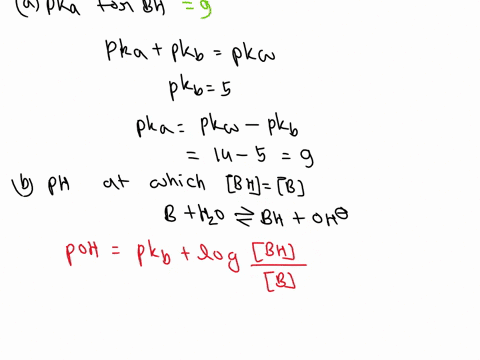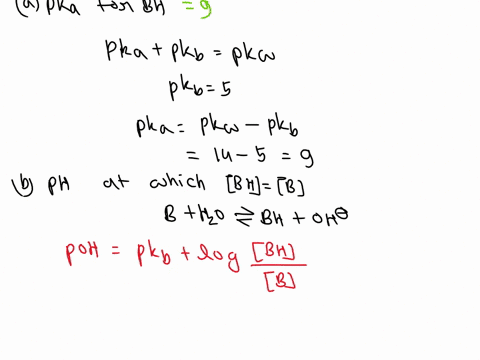
SOLVED: A diprotic base B has pKa values of 3.54, 7.08, and 10.46. Which of the equalities is true when the pH is 6.92? [BH+] = [B] [BH] = [B] [BH+] = [
The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points

PHARMACEUTICAL ANALYSIS I - ACID BASE TITRATIONS
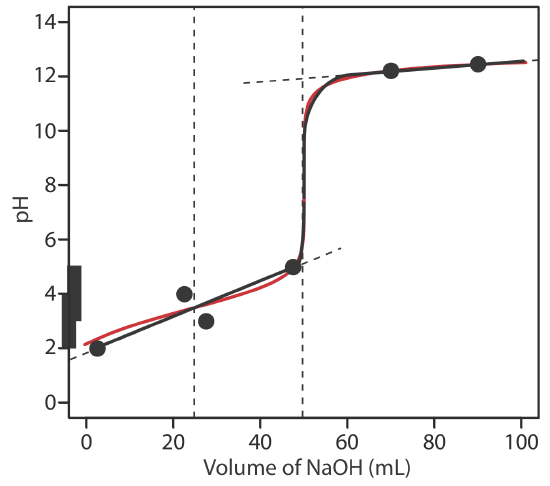
6.6: pH Calculations for Acid–Base Titrations - Chemistry LibreTexts

SOLVED: A diprotic base B has pKa values of 3.54, 7.08, and 10.46. Which of the equalities is true when the pH is 6.92? [BH+] = [B] [BH] = [B] [BH+] = [

Chemistry of the Non-Metals: Syntheses - Structures - Bonding - Applications 9783110578058, 9783110578065, 9783110578317

Equilibrium - Flip eBook Pages 51-100

Solved A diprotic base (B) has pKb values of 4.95(pKb1) and

An In-Depth Examination of Ionic Equilibria and Factors Affecting the Degree of Dissociation, PDF, Acid

Equilibrium - Flip eBook Pages 51-100
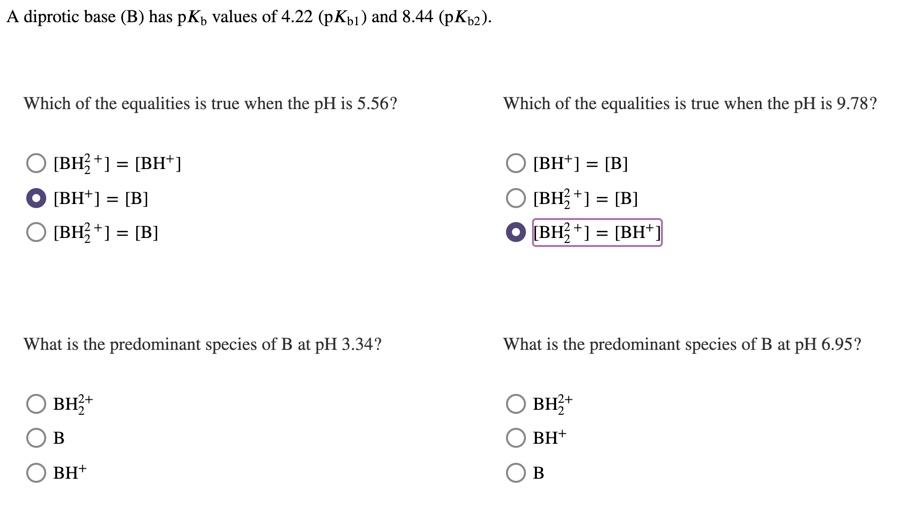
Solved A diprotic base (B) has pK) values of 4.22 (pKb) and
- Shapewear Bodysuit, Ice Silk Waist Slimmer Shapewear Lingerie Nylon Thin for Home for Female (L Suitable for 132.3-154.3lb) : : Clothing & Accessories

- Ultra LiftPants - Gerodan
- The Woman in The Red Bra is Lying on The Bed Poster Print Canvas Poster Wall Art Party Birthday Gifts Indoor Decorations Suitable for Family Dormitory Office Bathroom Decor: Posters

- Eda cashmere bralette in beige - Khaite

- Men's First Choice Fritz Handle - Black



