SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.

By A Mystery Man Writer
VIDEO ANSWER: We can say permanganate permanganate iron, which is here, or we can say intense, intense purple colorati. The oxidation state of the manganese can be found in the m n, o 4 negative. Here, we can say that it is plus 7. The electrons are
[MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2. d-d transitions in the Mn compound compared to the Re compound 3. charge transfer from O to Re compared to O to Mn 4. charge transfer from O to Mn compared to O to Re.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

009) Coordination Chemistry

coordination compounds - How can the intense color of potassium permanganate be explained with molecular orbital theory? - Chemistry Stack Exchange
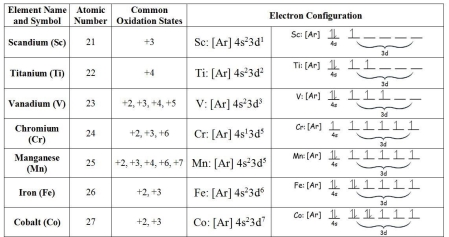
3.1 Transition Elements 2 Chemistry of Ti V Cr Mn Fe and Co in various oxidation states excluding their metallurgy
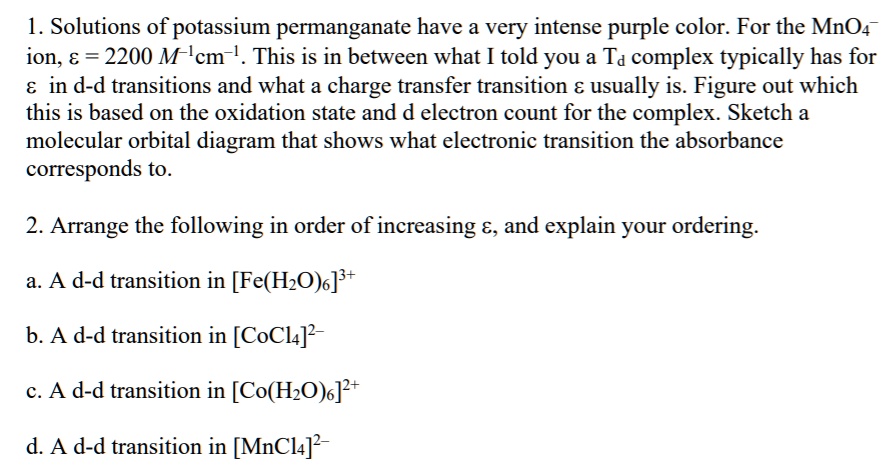
SOLVED: Solutions of potassium permanganate have a very intense purple color. For the MnO4 ion, € = 2200 M-1cm-1. This is in between what I told you a Td complex typically has

SOLVED: MnO4 is a stronger oxidizing agent than ReO4. Both ions have charge-transfer bands; however, the charge-transfer band for ReO4 is in the ultraviolet, whereas the corresponding band for MnO4 is responsible

The purple color of KMnO4 is due to:
3.1 Transition Elements 2 Chemistry of Ti V Cr Mn Fe and Co in various oxidation states excluding their metallurgy

SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.

Assertion: KMnO_(4) is purple in colour due to charge transfer . Reason :There is no electron pr

ReasonColour of KMnO_4 is due to charge transfer.AssertionKMnO_4 is a colourless compound.
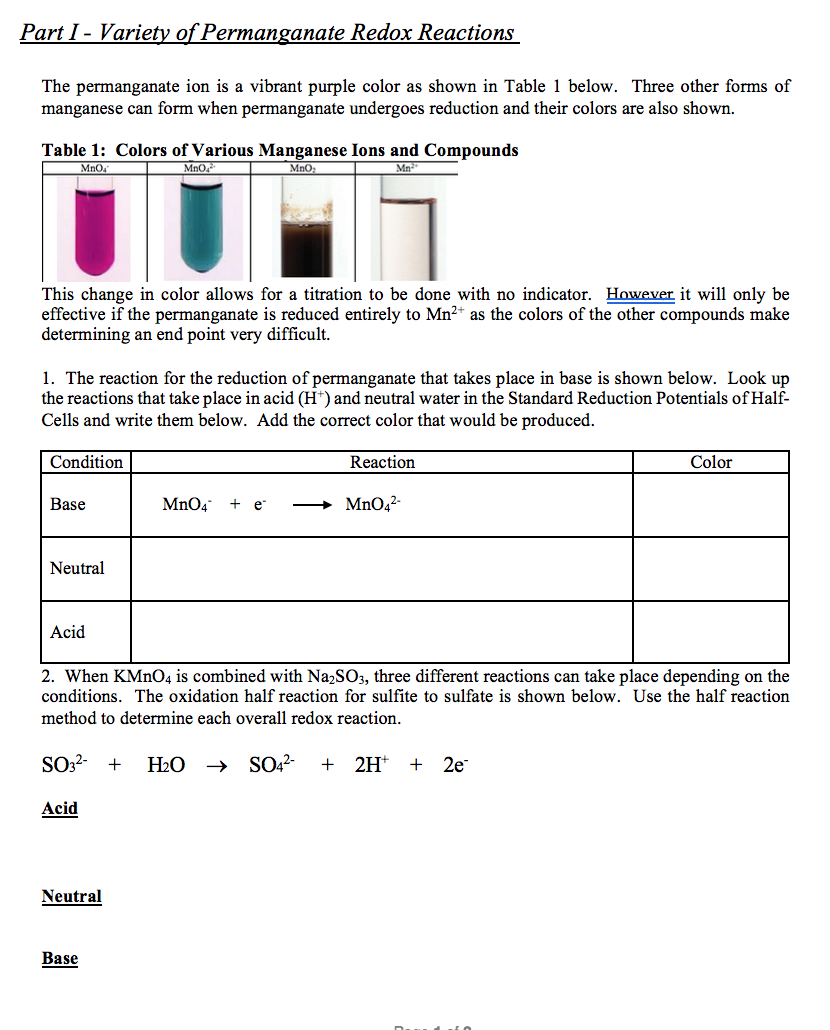
Solved Part I - Variety of Permanganate Redox Reactions The
Why is KMnO4 intensely coloured whereas KTeO4 and KReO4 are colourless? - Quora
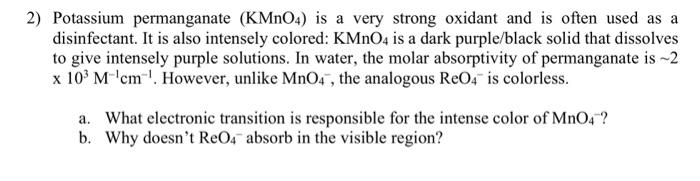
Solved 2) Potassium permanganate (KMnO4) is a very strong

PDF) Applications of Supramolecular Anion Recognition
- AGUUD-Rapid Volume Fans Lashes, C, CC, D, DD Curl, Extensões de cílios, Cílios Blooming, 0.03, 0.05, 0.07mm, Cílios Easy Fan

- Solidly between cup sizes. D or DD? : r/ABraThatFits

- 1 or 3 Bra 6 BRAS Cross BACK Open Front PLUNGE T-SHIRT 1836 Underwire LOT D DD

- What's the difference between Capital D Disabled and lowercase d disabled?, D&A

- Comparison between DD and ID. The first row shows the four morph

- The Best crochet stitches for sweaters - Fosbas Designs

- 208,377 Clothes Vector Stock Photos, High-Res Pictures, and Images
- VERSACE JEANS COUTURE, Lilac Women's Midi Dress

- Goddess Verity Full Cup Non Wire Bra (700218),40I,Ultramarine

- 1914-Allies-1918 Lapel Pin, National Symbols the Poppy & Le Bleuet - Lest We Forget UK
