NGAL - Bioporto
By A Mystery Man Writer
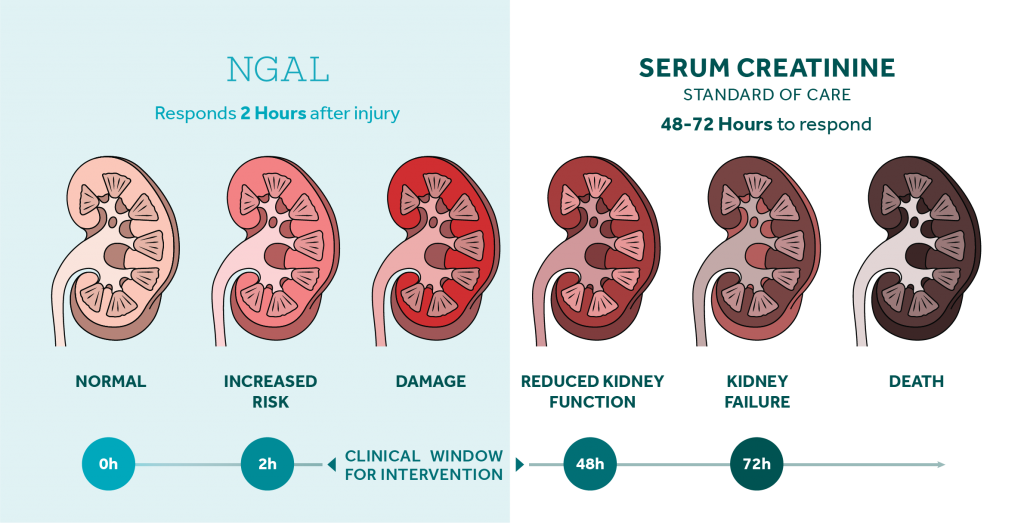
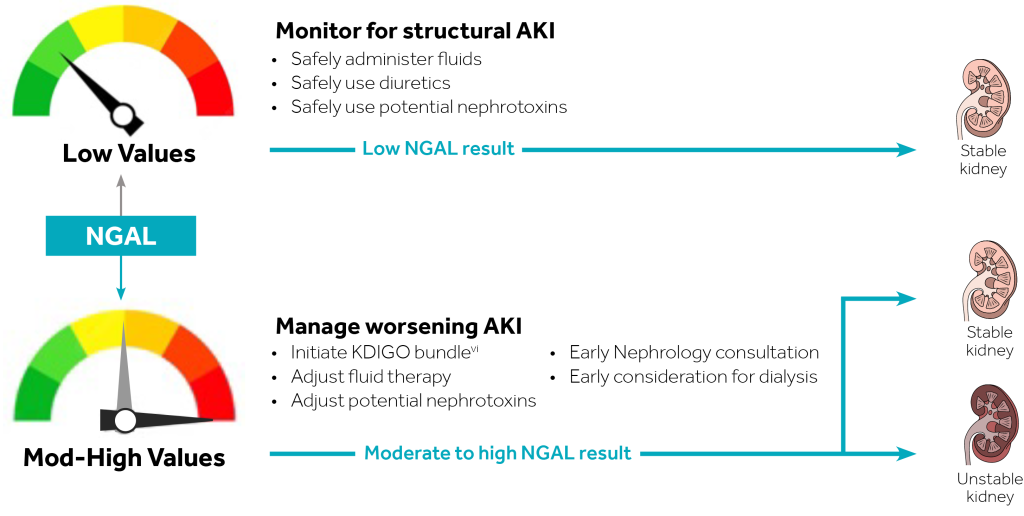
The NGAL Test is a particle-enhanced turbidimetric immunoassay for the quantitative determination of NGAL in human urine and plasma on automated clinical chemistry analyzers. NGAL measurements are useful in the risk assesment of AKI.

Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19 - ScienceDirect

The Prognostic Utility of Plasma NGAL Levels in ST Segment Elevation in Myocardial Infarction Patients
Clonezyme Biotek

May Neutrophil Gelatinase-Associated Lipocalin (NGAL) Level Predict Mortality in Patients with Hepatocellular Carcinoma (HCC)?
NGAL - Bioporto
IJMS, Free Full-Text

The effect of targeting Tie2 on hemorrhagic shock-induced renal perfusion disturbances in rats, Intensive Care Medicine Experimental
Home - Bioporto

BioPorto Diagnostics A/S: Contact Details and Business Profile

BioPorto Diagnostics A/S
- STOP Using A Lint RollerUse A Clothes Brush Instead!

- Buy Wholesale China Custom Logo Solid Color Spandex/polyester Sportswear High Waist Fitness Women's Athletic Leggings & Woman Legging Push Up Sweatpants at USD 5.99

- 34H Swim by Freya, Free Shipping

- Briefs Heart Print GPL Exotic Cotton Ladies Panties at Rs 48/piece in New Delhi

- Buy Plus Size Ultra Soft Churidar Cotton Lycra Regular Fit





