Applications for Medical Device Investigational Testing Authorizations Guidance Document

By A Mystery Man Writer
Applications for Medical Device Investigational Testing Authorizations Guidance Document

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Medical Device Guidelines and Regulations Handbook

/wp-content/uploads/120650

Applications for Medical Device Investigational Testing Authorizations Guidance Document

A Quick & Easy Guide to FDA Pre-Submissions
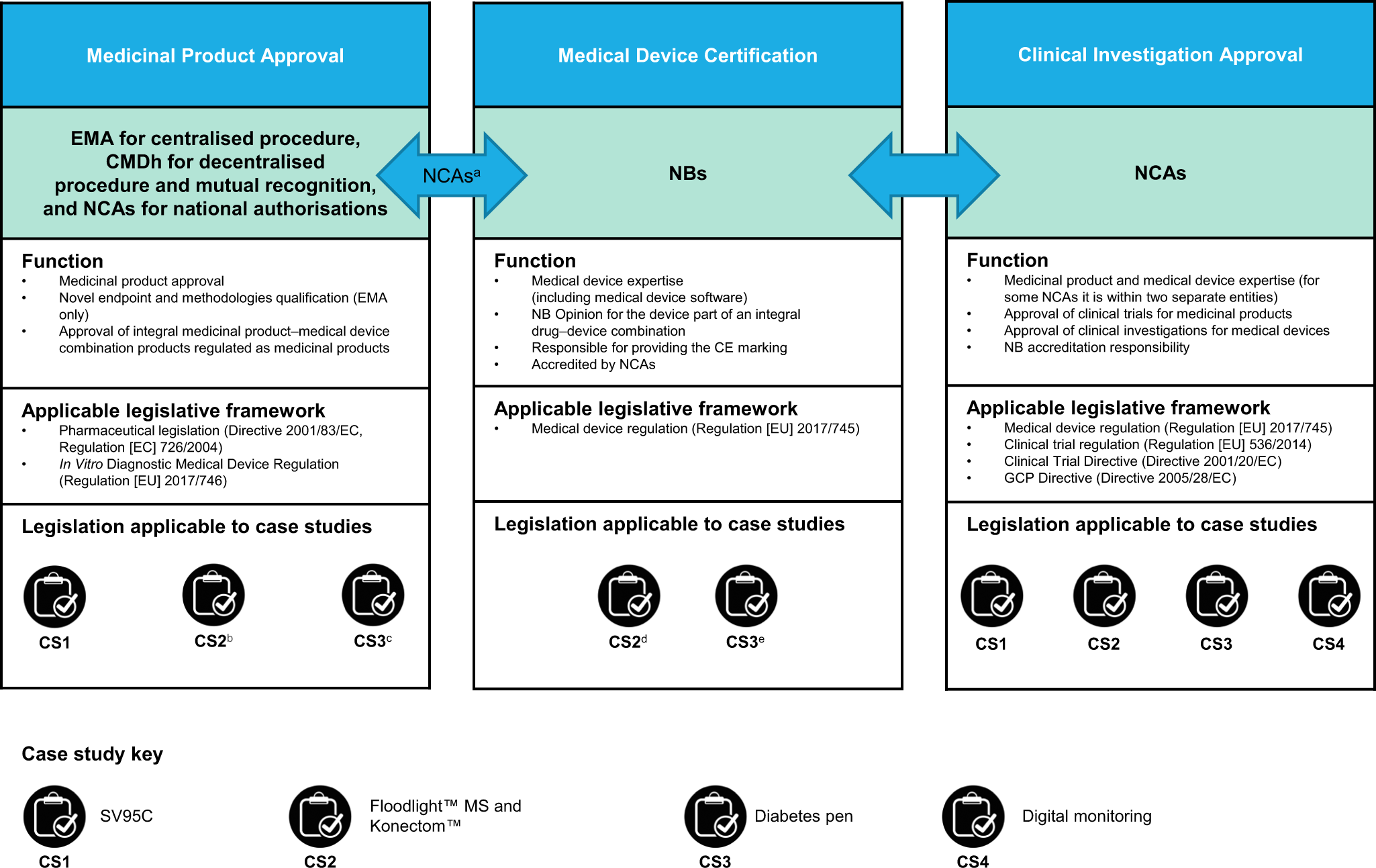
Evolving regulatory perspectives on digital health technologies

Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations

FDA Guidance on Dual 510(k) and CLIA Waivers
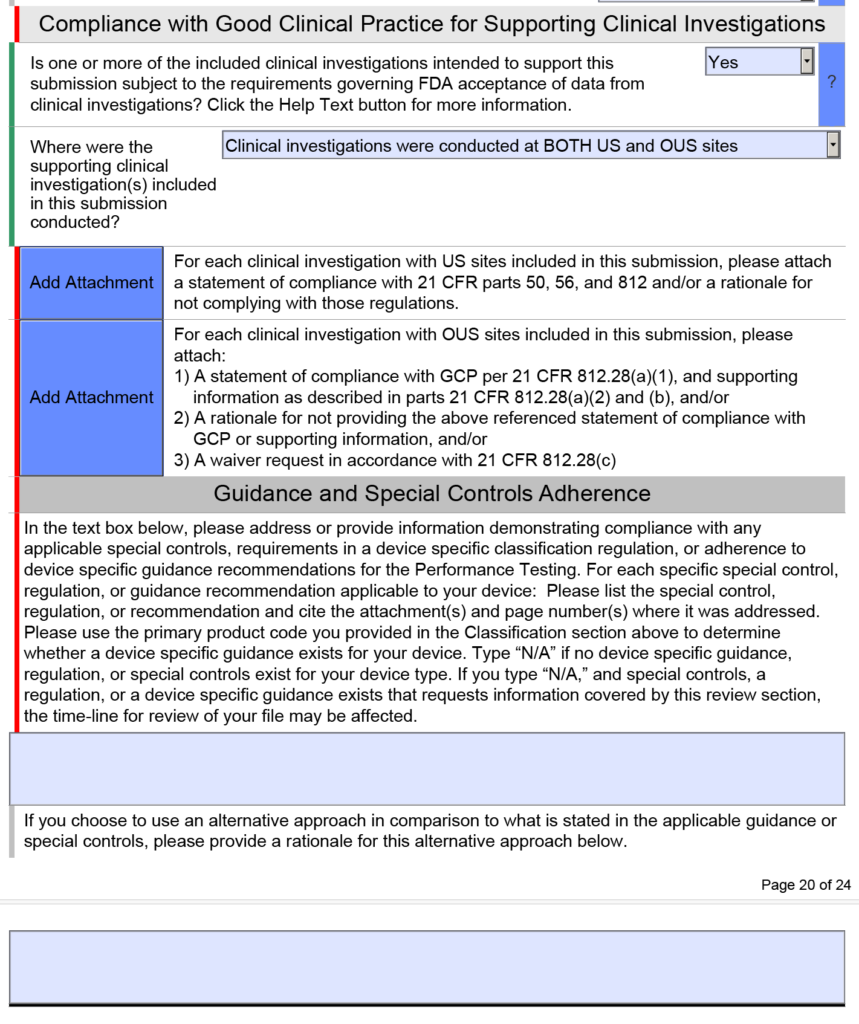
/wp-content/uploads/FDA-eS

Technical Writing for Medical Devices Freyr - Global Regulatory Solutions and Services Company

What Labs Need to Know About New Medical Device Cybersecurity Rules
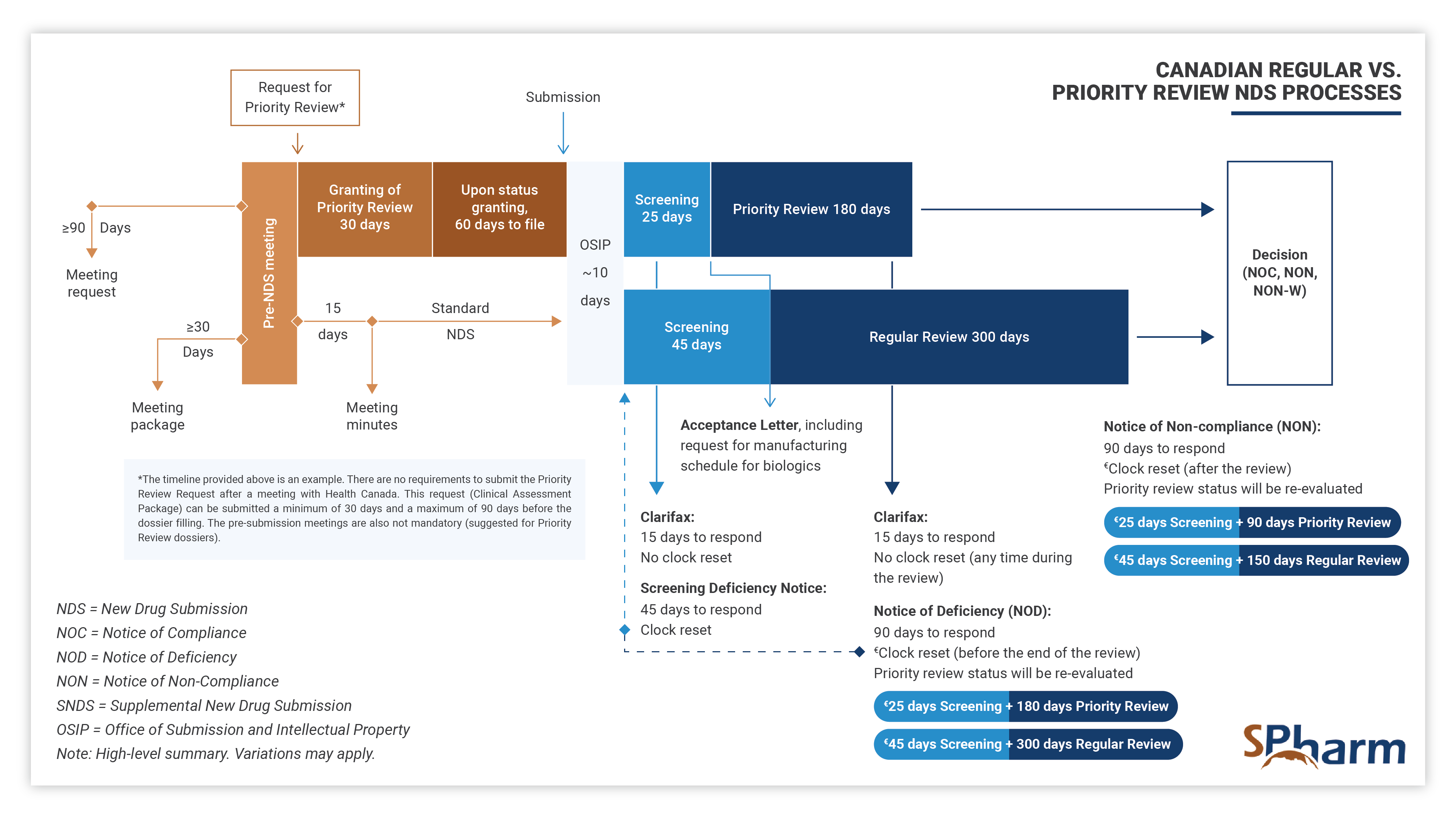
New Drug Submission Process in Canada
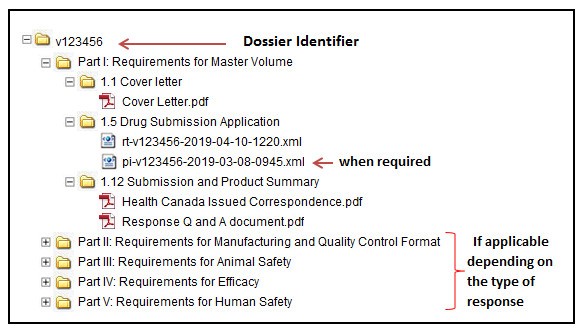
Guidance document: preparation of regulatory activities in non

Necessity of strengthening the current clinical regulatory for companion diagnostics: An institutional comparison of the FDA, EMA, and MFDS - ScienceDirect

FDA Guidance on Considerations for Weight Loss Devices: Study Design, Duration, and Follow-Up
- Buy Zivame Lace Embrace Strapless Long Line Push Up Bra- Red at Rs.1495 online

- Branded Sport-Tek Ladies Hooded Wind Jacket Black Heather/Black

- Playtex 18 Hour Active Breathable Comfort Wirefree Bra
- Pantalones Jeans De Dama, Jeans Strech Para Mujer

- Brand Bras For Women Great Breast 46 38 40 42 44 C D DD E Cup Bra