2.t 300 K, 36 g of glucose present per litre in itssolution has an
By A Mystery Man Writer
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would be itsconcentration?(1) 11 gl 1(3) 36 gl 1(2) 22 gL 1(4) 42 gL 1
2-t 300 K- 36 g of glucose present per litre in itssolution has an osmotic pressure of 4-98 bar- If theosmotic pressure of solution is 1-52 bar at thesame temperature- what would be itsconcentration-1- 11 gl-1-3- 36 gl-1-2- 22 gL-1-4- 42 gL-1
NCERT Solutions for Class 12 Chemistry Solutions

WICUS). GUJ-CET ld be the osmotic pressure of the system 300 k temperature ? (R=8.314 x 10-2 1 k-1) (Assume that lomic solid substances completely dissociates in an aqueous 7. What would
How many grams of glucose, C6H12O6, are necessary to prepare 598 ml of solution with concentration of 0.72 molar? - Quora

Short Answer Questions-II (PYQ), PDF, Solution

At 300K, 26g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar.
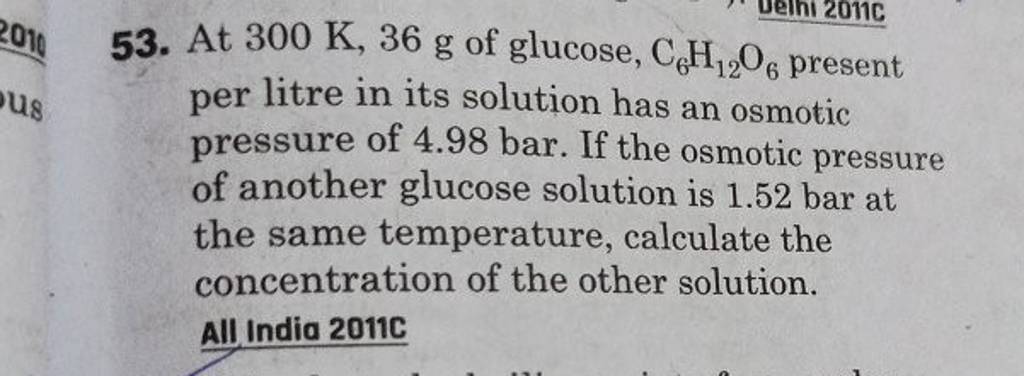
53. At 300 K,36 g of glucose, C6H12O6 present per litre in its solutio..
Class 12 Chemistry Chapter 2 NCERT Solutions
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions - CBSE Tuts

At 300 K,36 g of glucose present per litre in its solution has an osmotic..

MP Board Class 12th Chemistry Solutions Chapter 2 Solutions – MP Board Solutions

30 g of glucose present per litre has an osmotic pressure of 4.91 atm 303 K. If the osmotic pressure of the same solution is 1.5 atm the same tempera- ture, what









