At 300 K, 36 g of glucose present per litre in its solution has an osm

By A Mystery Man Writer
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

At 300 K, 36 gof glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars the same

The Hydrating Effects of Hypertonic, Isotonic and Hypotonic Sports Drinks and Waters on Central Hydration During Continuous Exercise: A Systematic Meta-Analysis and Perspective

Based on solute - solvent interactions, arrange the following in order

⏩SOLVED:At 300 K, 36 g of glucose present per litre in its…

Solved At 300 K, 36 g of glucose present in a litre of its

please explain the question and tell me what is 4 98 bar in this question and why it's not been - Chemistry - Solutions - 14451181
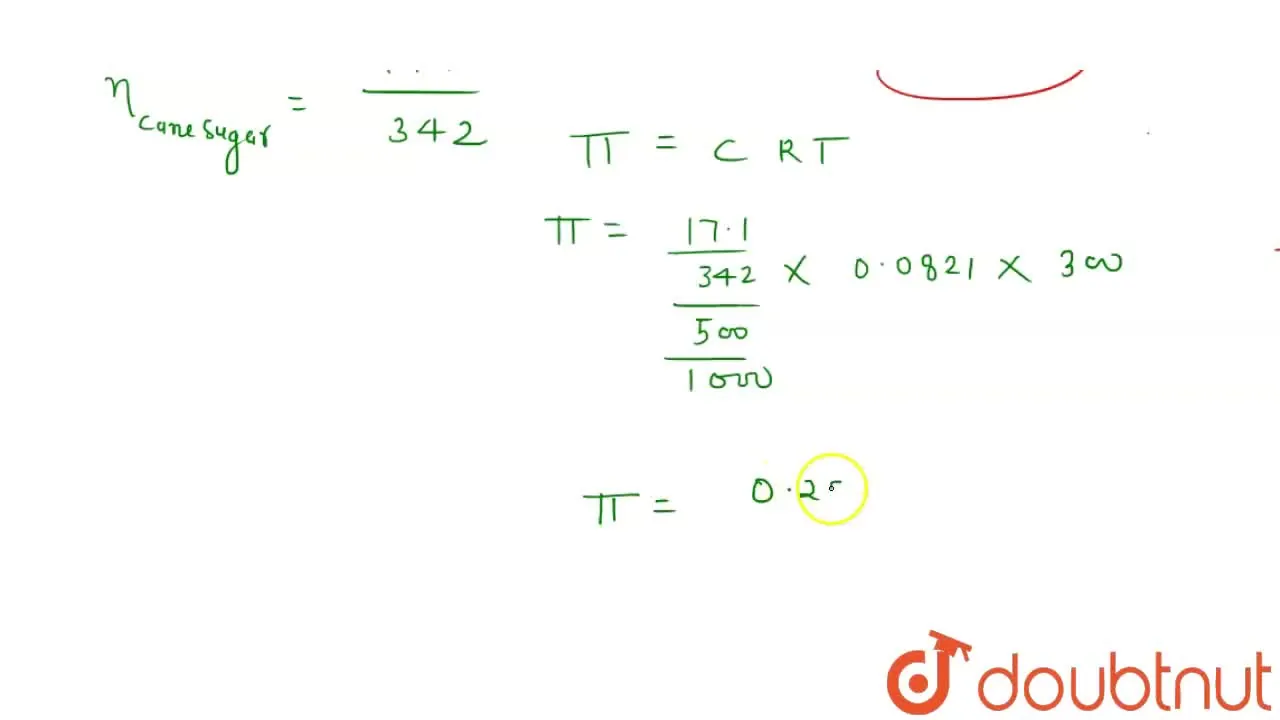
Calculate the osmotic pressure of a solution containing 17.1 g of cane

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

Osmolarity, Definition, Units & Calculations - Lesson
- 1) 2 23 g ethanol is dissolved in 36 g water. Find mole fraction

- Preventive Measures for Nuclear and Other Radioactive Material out

- Salame Sweet Chilli Sadia Salamitos 36g - giassi - Giassi

- Salame Sadia Pocket Salamitos 36g - mobile-superprix

- 1) 2 23 g ethanol is dissolved in 36 g water. Find mole fraction of ethanol (2) 0.5 (3) 0.2 (4) 0.8 TIN

- Capris for Women Casual Summer Cropped Pants Solid Wide Leg Pants Loose Elastic Waist Pants Baggy Capri Pants Polyester

- Silhouette of deer standing on field during sunset photo – Free
- Young Woman Practicing Yoga On The Beach At Sunset. Banco de Imagens Royalty Free, Ilustrações, Imagens e Banco de Imagens. Image 40866670.

- Sorella wireless bra & sport bra, Women's Fashion, New

- ADELE Fabric chair By JMS

