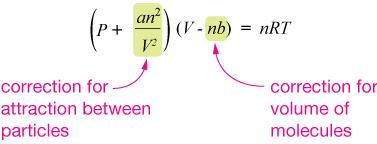Thursday, Sept 19 2024
Ideal–Universal Gas Law
By A Mystery Man Writer
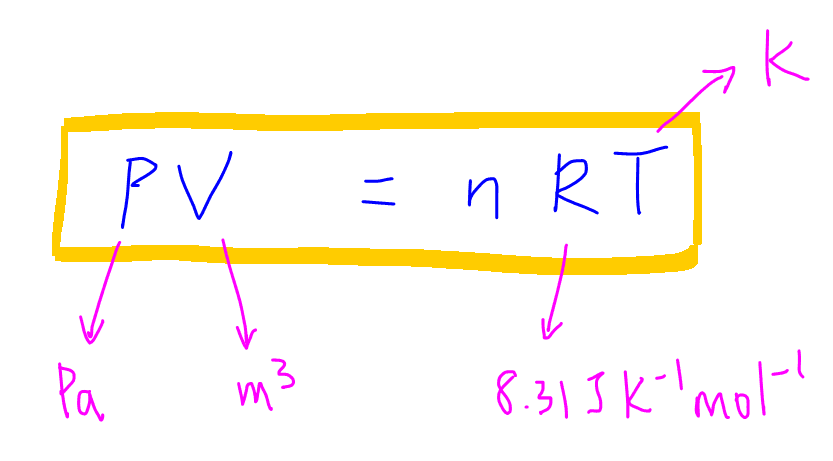
Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.” NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K Relation: The relation between pressure (P) volume (V), number of moles (n) and…
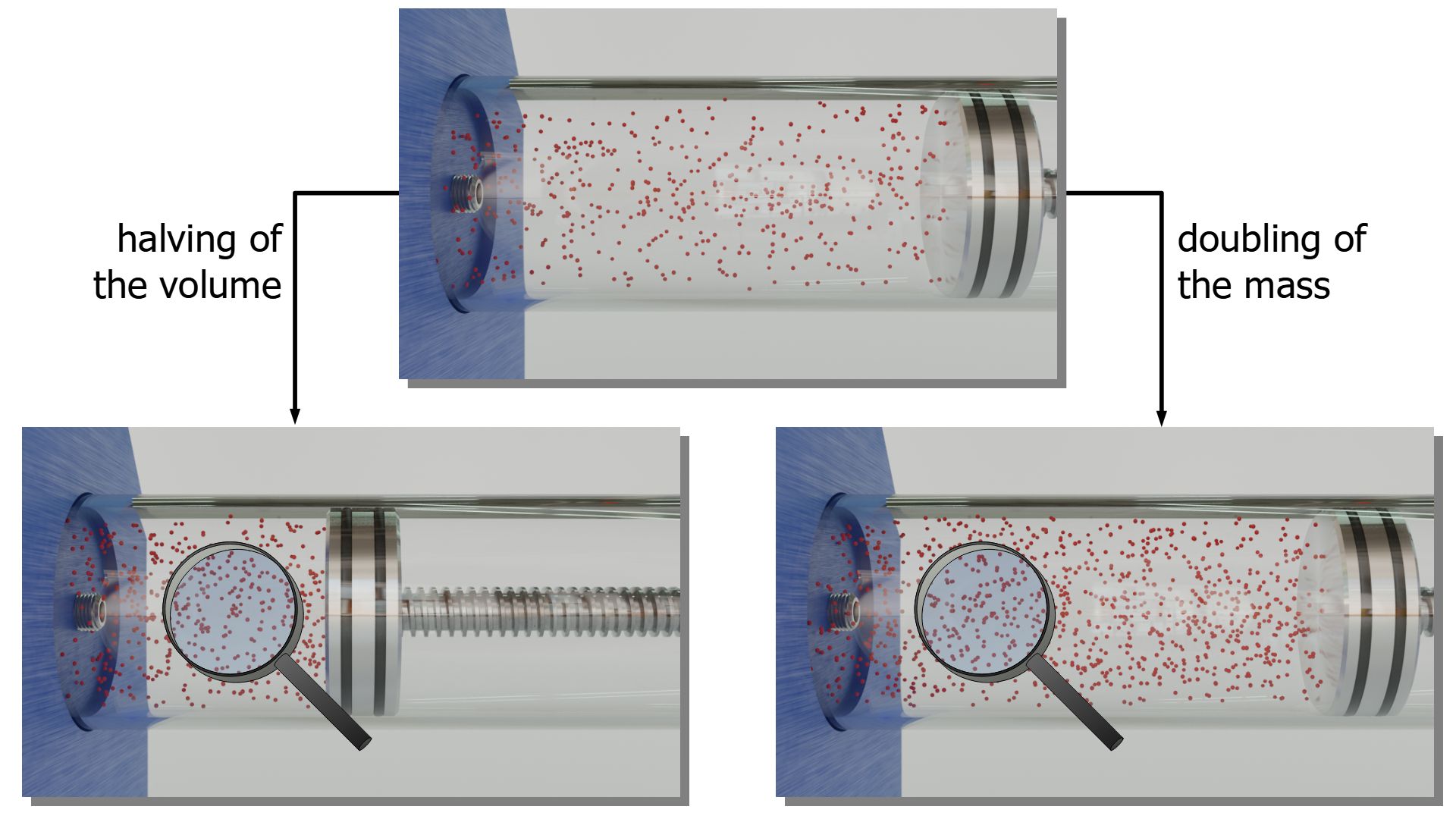
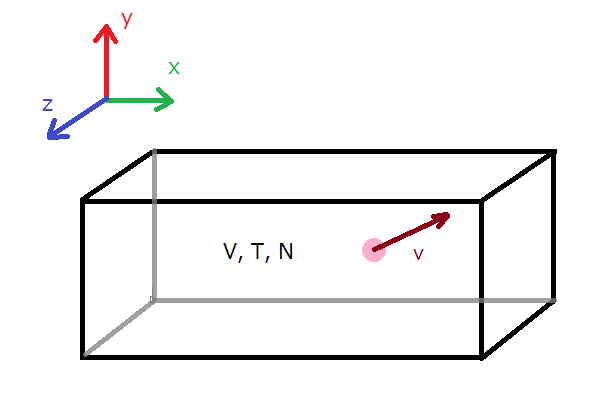
Ideal gas law (explained and derived) - tec-science

Polarity: Dipoles and Dipole Moments

The Activity Series

Electromagnetic Spectrum
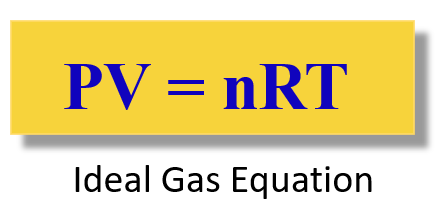
The Ideal Gas Law - Chemistry Steps
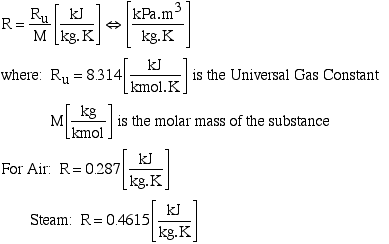
Chapter 2b: Pure Substances: Ideal Gas (updated 1/17/11)
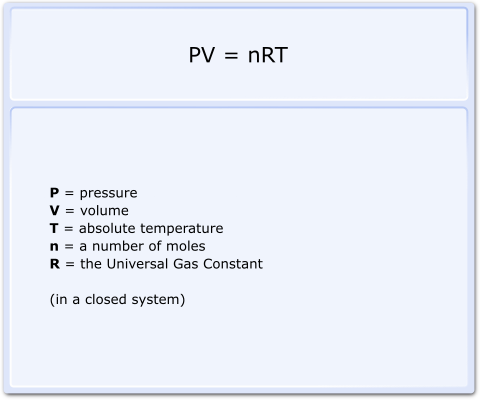
ANA14_01_020_09_02_50.png
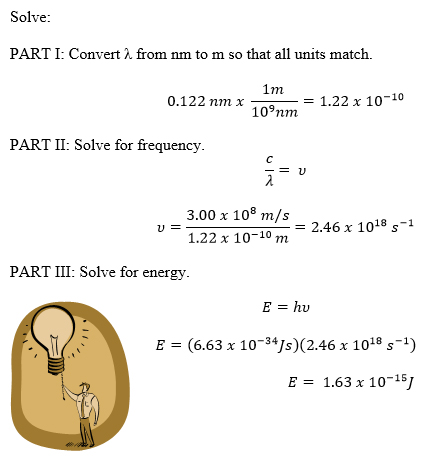
Plank's Black Body Equation
:max_bytes(150000):strip_icc()/200175879-001-56a12e6b5f9b58b7d0bcd67f.jpg)
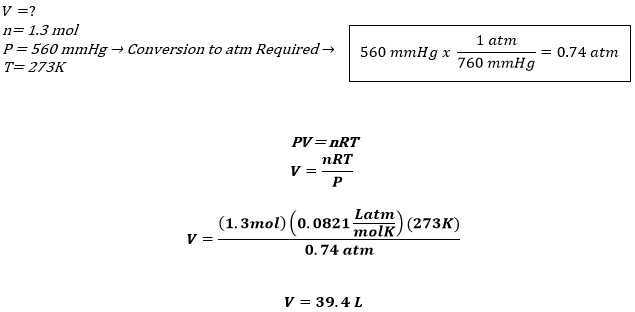
An Explanation of the Ideal Gas Law
Related searches
©2016-2024, reintegratieinactie.nl, Inc. or its affiliates