Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

By A Mystery Man Writer
Results of the experimental measurements on the partial molar volume of kerosene used as a medium for dissolving TBP are utilized to determine the activity of TBP in the binary kerosene-TBP solution through the application of Gibbs-Duhem equation. The treatment is based on combination of the experimental data with the thermodynamic values available on the compressibility factor of pure kerosene at room temperature. It is shown that the activity of TBP in kerosene has a positive deviation from ideality with an activity coefficient derived as follows:1) at X TBP ≤ 0.01: γ TBP = 42.530, 2) at the 0.01 X TBP 0.2: 3) at the higher TBP concentrations 0.2 X TBP 0.97: and 4) at TBP Raoultian concentrations 0.97 ≤ X TBP:γ TBP = 1. These quantities can be utilized at temperature closed to 298 K.

McCabe-Thiele plot for stripping of manganese loaded D2EHPA using

Water adsorption in the organic phase for the D2EHPA-kerosene

Solvent Extraction of Nickel and Zinc from Nitric Acid Solution

Water adsorption in the organic phase for the D2EHPA-kerosene

PDF) Thermodynamics of extraction of Zn2+ from sulfuric acid media

Solvent Extraction of Nickel and Zinc from Nitric Acid Solution Using D2EHPA: Experimental and Modeling

PDF) Developing a Thermodynamical Method for Prediction of

Separation of Re and Mo from Roasting-Dust Leach-Liquor Using

Separation of Re and Mo from Roasting-Dust Leach-Liquor Using

PDF) Solvent Extraction: Classical and Novel Approaches This page intentionally left blank Solvent Extraction: Classical and Novel Approaches

Solvent Extraction of Nickel and Zinc from Nitric Acid Solution Using D2EHPA: Experimental and Modeling

Separation of Re and Mo from Roasting-Dust Leach-Liquor Using
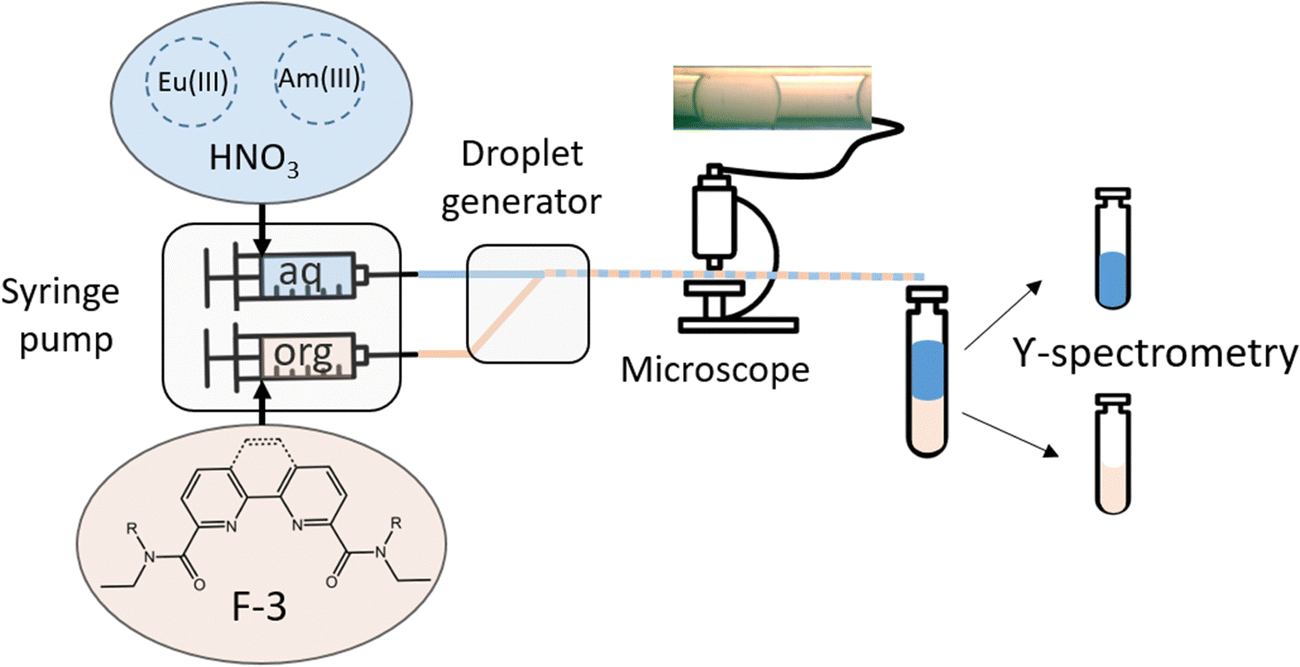
Kinetic features of solvent extraction by N,O-donor ligands of f-elements: a comparative study of diamides based on 1,10-phenanthroline and 2,2′-bipyr - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D3CP05081E

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

Developing a Thermodynamical Method for Prediction of Activity
- ChemE 260 Equations of State April 4, 2005 Dr. William Baratuci Senior Lecturer Chemical Engineering Department University of Washington TCD 2: E & F CB. - ppt download

- At certain states, the p-v-T data of a gas can be expressed
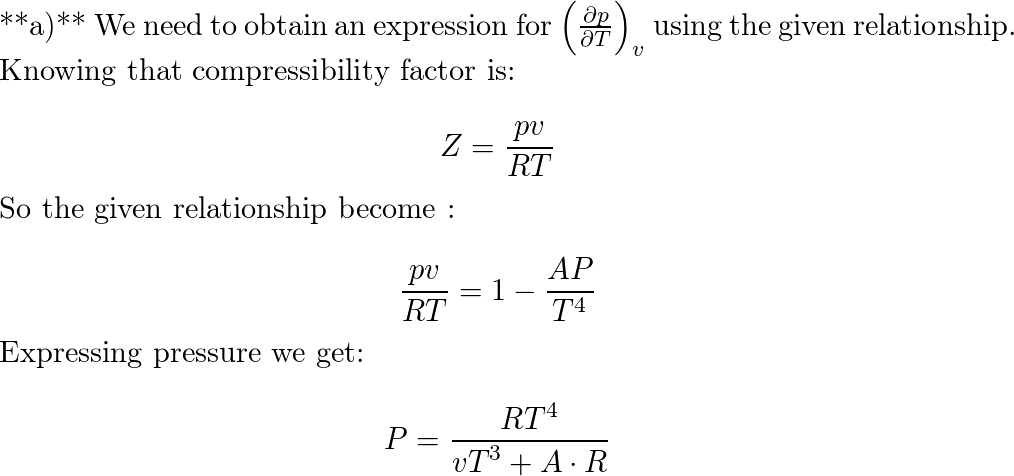
- Qin Lab - thermal data
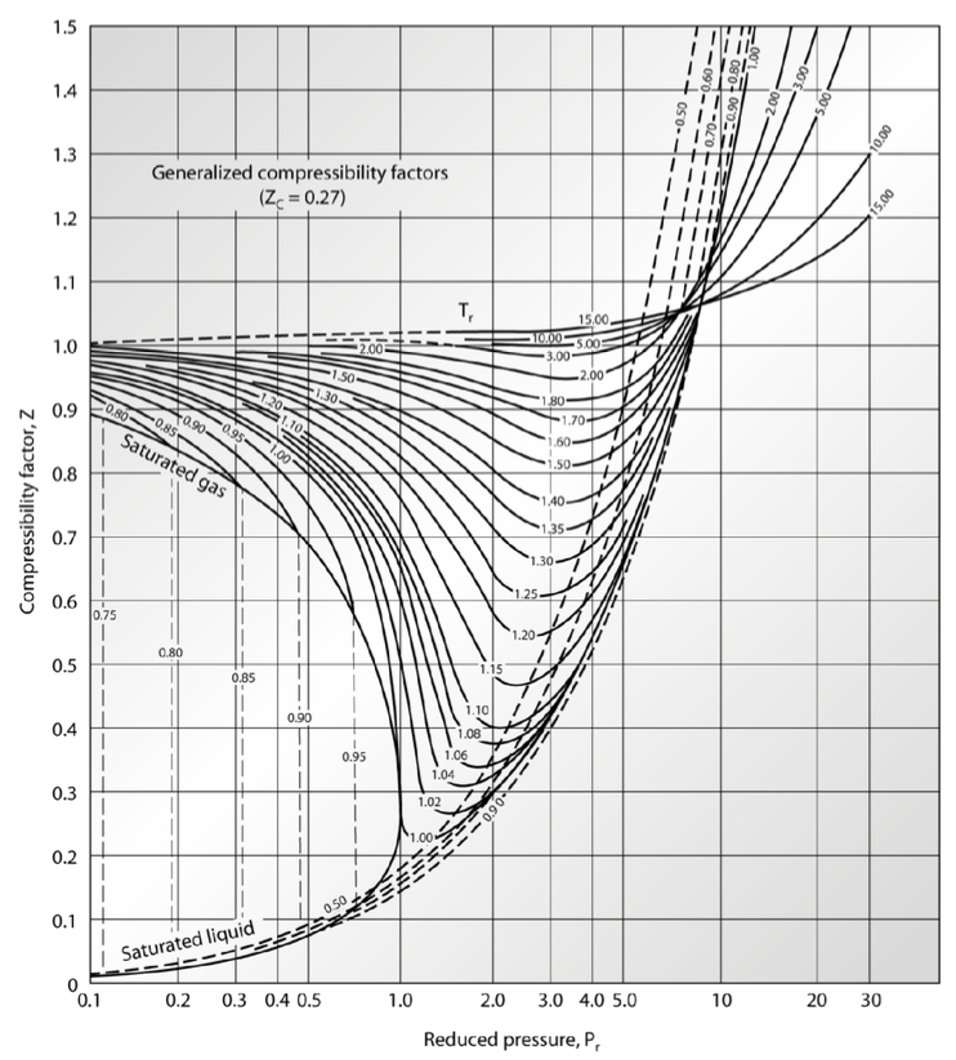
- Solved] Why is the compressibility factor less than 1 at most
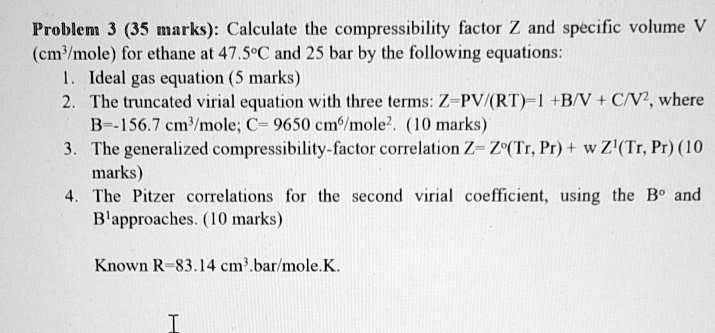
- SOLVED: Problem 3 (35 marks): Calculate the compressibility factor Z and specific volume V cm/mole for ethane at 47.5°C and 25 bar by the following equations: 1. Ideal gas equation - 5
- Men's Y2K Clothes Tracksuits Track Baggy Cargo Pants Vintage Oversize Clothing Joggers Harajuku Streetwear Sweatpants Black Wide Trousers For Men

- Cathalem Women's One Fab Fit Underwire Bra Smoothing Bliss Wireless Lightly Lined Convertible Comfort Bra,Beige 85

- Cute Sweet Plush Bear Print Bra And Panty Set Winter Pure Desire

- America's M4 Sherman Tank, a WWII War Machine
:max_bytes(150000):strip_icc()/A_Sherman_tank_of_8th_Armoured_Brigade_in_Kevelaer_Germany_4_March_1945._B15145-5ee92fd41e4040bab305638b2f6b8fcf.jpg)
- Discretos pero muy absorbentes: los 5 mejores pañales para adultos
