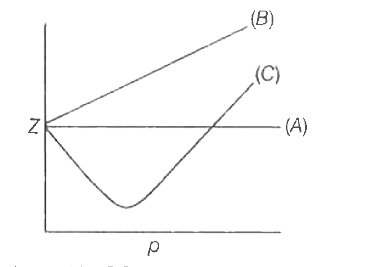
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

By A Mystery Man Writer

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

EngArc - L - Compressibility Factor

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for ACT PDF Download

COMPRESSIBILITY FACTOR
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

Gas—General - ScienceDirect
Telugu] The variation of compressibility factor (Z) with pressure (p

Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

Compressibility factor - Wikipedia
- Bedsure Sage Green Duvet Cover Queen Size - Soft Prewashed Queen Duvet Cover Set, 3 Pieces, 1 Duvet Cover 90x90 Inches with Zipper Closure and 2

- Buy Below Knee Compression Stockings online
- Logo Tank

- Lululemon black grape emerge renewed jacket stained glass love nightfall speed tights - Agent Athletica

- LEGO Peça - Toothed Bar Z10 Gear Rack 1x4 (White) - Portugal




