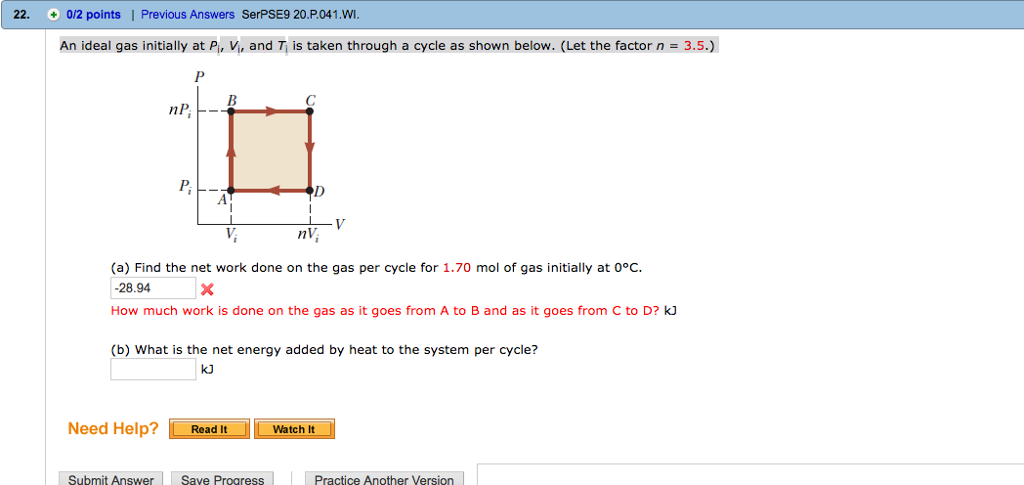
An ideal gas initially P_i ,V_i , and T_i is taken through a cycle

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:an ideal gas initially at pi vi and ti is taken through a cycle
Click here👆to get an answer to your question ✍️ An ideal gas initially P-i -V-i - and T-i is taken through a cycle as shown in Figure- -a- Find the net work done on the gas per cycle 1-00 mol of gas initially 0-0C- -b- What is the net energy added by heat to the gas per cycle
Isothermal Compression of a Ideal Gas and Distance
Solving a Monatomic Ideal Gas Expansion Problem

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

11/14/2013PHY 113 C Fall Lecture 221 PHY 113 C General Physics I 11 AM – 12:15 PM MWF Olin 101 Plan for Lecture 22: Chapter 21: Ideal gas equations. - ppt download
Solved An ideal gas initially at Pi, Vi, and Ti is taken

An ideal gas initially at pressure P0, volume V0, and temperature T0 is taken through the cycle described in Figure P12.54, with n = 4 and m = 3. Figure P12.54 (a)

Process integration, energy and exergy analyses of a novel integrated system for cogeneration of liquid ammonia and power using liquefied natural gas regasification, CO2 capture unit and solar dish collectors - ScienceDirect

SOLVED: 3P; Pi A V 3V; A 1 mole of ideal gas initially at Pi-l Pa, Vi–5 m, and Ti= 0°C is taken through a cycle as shown in the above Figure.

which is not really negligible with respect to unity Therefore the complete